Zearalenone
Synonym(s):(3S,11E)-3,4,5,6,9,10-hexahydro-14,16-dihydroxy-3-methyl-,1H-2-benzoxacyclotetradecin-1,7(8H)-dione;F-2 toxin;ZON
- CAS NO.:17924-92-4
- Empirical Formula: C18H22O5
- Molecular Weight: 318.36
- MDL number: MFCD00133085
- EINECS: 241-864-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 08:49:36
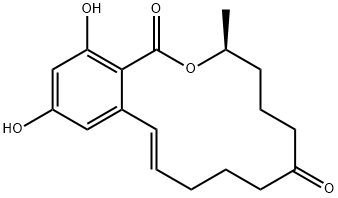
What is Zearalenone?
Chemical properties
Crystalline Solid
The Uses of Zearalenone
Zearalenone is a resorcylic acid lactone produced by a number of Fusarium sp.. Zearalenone acts as a non-steroidal estrogen, binding to estrogen receptor and is uterotropic. Zearalenone induces reproductive problems in animals and, in some animal models, is thought to be a primary initiator of hepatic tumours. In vivo, zearalenone undergoes metabolic reduction to the more estrogenic zearalenol. Contamination of grains, notably maize, by Fusarium species gives rise to high levels of zearalenone and is regarded as an important food quality issue for both humans and animal health.
The Uses of Zearalenone
Estrogenic mycotoxin produced by Fusarium fungi commonly found in grains. One of a group of compounds known as resorcylic acid lactones
The Uses of Zearalenone
A phytoestrogenic mycotoxin and ER activator
What are the applications of Application
Zearalenone is a phytoestrogenic mycotoxin and ER activator
Definition
ChEBI: A macrolide comprising a fourteen-membered lactone fused to 1,3-dihydroxybenzene; a potent estrogenic metabolite produced by some Giberella species.
General Description
White microcrystals or white powder.
Air & Water Reactions
Zearalenone may be sensitive to prolonged exposure to air. Insoluble in water.
Reactivity Profile
Zearalenone is an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides. Zearalenone may be incompatible with alkalis.
Fire Hazard
Flash point data for Zearalenone are not available. Zearalenone is probably combustible.
Biochem/physiol Actions
Zearalenone, a fungal mycotoxin produced by Fusarium, binds the estrogen receptor (ER) and is uterotropic in the newborn rat. It is a common contaminant in cereal grain used for animal and human food, and exerts an estrogenic activity that modulates/disrupts endocrine function in animals and possibly humans.
Metabolic pathway
When zearalenone is subjected to microbial transformation by a fungus, Gliocladium roseum, it is converted to a 1 : 1 mixture of 1-(3,5-dihydroxyphenyl)- 10 0 -hydroxy-1-undecen-6'-one and 1-(3,5- dihydroxyphenyl)-6'-hydroxy-1-undecen-10'-one.
Storage
-20°C
Properties of Zearalenone
| Melting point: | 164-165°C |
| Boiling point: | 377.53°C (rough estimate) |
| alpha | 25546 -170.5° (c = 1.0 in CH3OH) |
| Density | 1.1270 (rough estimate) |
| refractive index | 1.6120 (estimate) |
| Flash point: | 6 °C |
| storage temp. | 2-8°C |
| solubility | DMF: 30 mg/ml; DMSO: 30 mg/ml; DMSO:PBS (pH 7.2) (1:7): 0.12 mg/ml; Ethanol: 20 mg/ml |
| form | Solid |
| pka | 7.58±0.40(Predicted) |
| form | neat |
| color | Off-white |
| Merck | 13,10169 |
| BRN | 1350216 |
| CAS DataBase Reference | 17924-92-4(CAS DataBase Reference) |
| EPA Substance Registry System | Zearalenone (17924-92-4) |
Safety information for Zearalenone
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Health Hazard GHS08 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Zearalenone
| InChIKey | MBMQEIFVQACCCH-QBODLPLBSA-N |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Zearalenone solution CAS 17924-92-4View Details
Zearalenone solution CAS 17924-92-4View Details
17924-92-4 -
 Zearalenone solution CAS 17924-92-4View Details
Zearalenone solution CAS 17924-92-4View Details
17924-92-4 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
