Triptorelin
Synonym(s):[D -Trp6]-Luteinizing hormone releasing hormone
- CAS NO.:57773-63-4
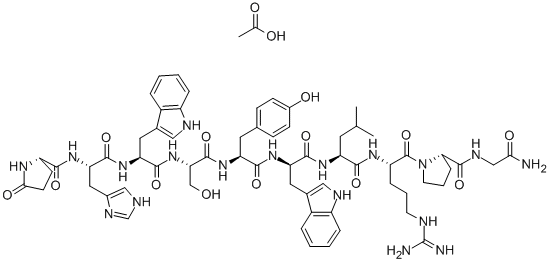
- Empirical Formula: C64H82N18O13
- Molecular Weight: 1311.45
- MDL number: MFCD00167541
- EINECS: 637-328-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-03-20 19:16:41
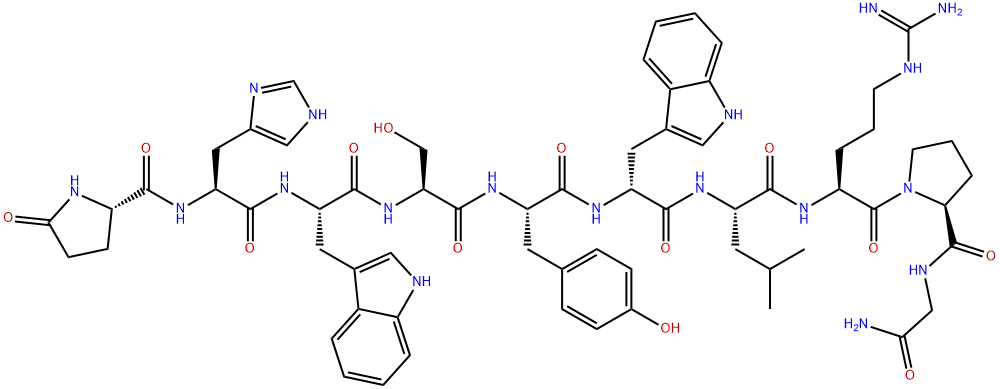
What is Triptorelin?
Absorption
Following IV administration of triptorelin, triptorelin is completely absorbed.
Toxicity
Some of the most commonly reported adverse effects of triptorelin are hot flushes reported in 58.6% of patients, skeletal pain in 12.1%, impotence in 7.1%, and headache in 5.0%. Other reported adverse effects include injection site pain, general body pain, leg pain, fatigue, hypertension, dizziness, diarrhea, vomiting, insomnia, emotional lability, anemia, pruritus, urinary tract infections, and urinary retention. Triptorelin is classified as Pregnancy Category X and contraindicated in pregnant women or in women who may become pregnant. Hormonal changes caused by triptorelin increase the risk for pregnancy loss. Studies done on pregnant rats demonstrated maternal toxicity and embryo-fetal toxicities.
Description
Decapeptyl is a modified (D-Trp6) LH-RH. Like recently marketed buserelin and leuprolide (I), it is useful in achieving medical castration in the treatment of advanced prostate cancer.
Originator
Tulane Univ. (USA)
The Uses of Triptorelin
Triptorelin is a GnRH agaonist shown to inhibit estradiol-induced cancer cell proliferation.
The Uses of Triptorelin
promoting ovulation
The Uses of Triptorelin
[D-Trp6]-LH-RH (luteinizing hormone releasing hormone) has been used to study whether the (M. – X) region in electron capture dissociation provides information on amino acid composition of polypeptides. It is also used to study the effect of LH-RH on the production of progesterone (P), estradiol (E2) or human chorionic gonadotropin (hCG) by JEG-3 choriocarcinoma cells.
Background
Triptorelin is a synthetic decapeptide agonist analog of luteinizing hormone releasing hormone (LHRH). Possessing greater potency than endogenous LHRH, triptorelin reversibly represses gonadotropin secretion. After chronic, continuous administration, this agent effects sustained decreases in LH and FSH production and testicular and ovarian steroidogenesis. Serum testosterone concentrations may fall to levels typically observed in surgically castrated men.
Indications
Triptorelin is indicated for the palliative treatment of advanced prostate cancer.
What are the applications of Application
Triptorelin is Triptorelin is a GnRHR agonist shown to inhibit estradiol-induced cancer cell proliferation.
Definition
ChEBI: An oligopeptide comprising pyroglutamyl, histidyl, tryptophyl, seryl, tyrosyl, D-tryptophyl, leucyl, arginyl, prolyl and glycinamide residues joined in sequence. It is an agonist analogue of gonadotropin-releasing hormone.
brand name
Trelstar (Watson).
Biochem/physiol Actions
Potent LH-RH agonist with enhanced biological activity due to its slower rate of degradation. Like [D-Lys6]-LH-RH, the D-Trp6 analog has been shown to be effective against cancers expressing the LH-RH receptor. However, unlike the D-Lys6 analog, it is generally used in the unconjugated form.
Pharmacokinetics
The first administration of triptorelin is followed by a transient surge of follicle stimulating hormone (FSH), luteinizing hormone (LH), estradiol,and testosterone. The time, peak and decline of testosterone in the body varies depending on the dose administered. This initial surge is often responsible for worsening of prostate cancer symptoms such as urethral or bladder outlet obstruction, bone pain, spinal cord injury and hematuria in the early stages. A sustained decrease in FSH and LH, and significant reduction of testicular steroidogenesis is usually seen 2-4 weeks post-initiation of therapy. This result is a reduction of serum testosterone to levels which are typically seen in surgically castrated men. Ultimately, tissues and functions that require these hormones become inactive. The effects of triptorelin can usually be reversed once the drug is discontinued.
Clinical Use
Triptorelin pamoate is another superagonist of GnRH, which like nafarelin acetate contains only a single amino acid substitution (D-Trp6 for Gly6) when compared to the natural hormone. In the treatment of advanced prostate cancer, it is important to reduce serum testosterone levels to very low levels, which can be achieved surgically by orchiectomy. When this surgical method is unacceptable to the patient, an alternative approach is “chemical castration,” which can be achieved by use of estrogen therapy, leuprolide,goserelin or histrelin acetates, and now, triptorelin pamoate. This product is available for IM depot injection (monthly or every 3 months), wherein serum testosterone concentration drops to a level generally seen in surgically castrated men.
Side Effects
The side effects of Triptorelin: Painful or difficult urination, burning when you urinate, blood in the urine; bone pain; (in children) new or worsening signs of puberty; a seizure; chest pain or pressure, pain spreading to your jaw or shoulder; sudden numbness or weakness, slurred speech; increased pressure inside the skull - severe headaches, ringing in your ears, dizziness, nausea, vision problems, pain behind your eyes; loss of movement in any part of your body; high blood sugar - increased thirst, increased urination, hunger, dry mouth, fruity breath odor; or nerve problems - back pain, muscle weakness, problems with balance or coordination, severe numbness or tingling in your legs or feet, loss of bladder or bowel control.
Metabolism
The metabolism of triptorelin in humans is not well understood; however, metabolism likely does not involve hepatic enzymes such as cytochrome P450. Whether or not triptorelin affects, or how it affects other metabolizing enzymes is also poorly understood. Triptorelin has no identified metabolites.
Metabolism
The metabolism of triptorelin in humans is unknown, but it is thought to be hydrolysed in the plasma and excreted in the urine as inactive metabolites.
Mode of action
Triptorelin is a gonadotrophin releasing hormone (GnRH) blocker. This means it stops messages from a part of the brain called the hypothalamus that tells the pituitary gland Open a glossary item to produce luteinising hormone. Luteinising hormone tells the testicles to produce testosterone Open a glossary item. So, blocking GnRH stops the testicles producing testosterone. Prostate cancer depends on testosterone to grow. So triptorelin can shrink the cancer or slow its growth. In women, it stops the ovaries Open a glossary item from producing oestrogen. Some breast cancers depend on oestrogen to grow. Lowering the level of oestrogen can slow or stop the growth of the cancer.
Properties of Triptorelin
| Melting point: | >180°C (dec.) |
| alpha | D23 -58.8° (c = 0.33 in acetic acid) |
| Density | 1.52±0.1 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | Aqueous Base (Slightly), Methanol (Very Slightly, Heated, Sonicated), Water (Sli |
| form | powder |
| pka | 9.82±0.15(Predicted) |
| color | White to Off-White |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 57773-63-4(CAS DataBase Reference) |
Safety information for Triptorelin
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H360:Reproductive toxicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Triptorelin
| InChIKey | HPPONSCISKROOD-OYLNGHKZSA-N |
New Products
3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-Iodophenylacetic acid Cyclohexane, (2-propynyloxy)- (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine Pivalic anhydride,98% Phenylmethanesulfonyl fluoride, 98% Glyoxylic acid solution, 50% in water tert-Butyl glycinate,97% 4-Ethoxybenzoic acid, 99% Sodium 1-octanesulfonate monohydrate 7-Ethyl Tryptophol 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl N, N-Carbonyldiimidazole (CDI) 5-Cyanophthalide 10-Methoxy-5H-dibenz[b,f]azepine 3-Methoxybenzonitrile Dibenzoyl Peroxide 4-Methoxybenzonitrile Titanium Dioxide Chloral PentachlorobenzonitrileRelated products of tetrahydrofuran




You may like
-
 57773-63-4 Triptorelin 98%View Details
57773-63-4 Triptorelin 98%View Details
57773-63-4 -
 57773-63-4 98%View Details
57773-63-4 98%View Details
57773-63-4 -
![[D-Trp6]-LH-RH CAS 57773-63-4](https://img.chemicalbook.in//Content/image/CP5.jpg) [D-Trp6]-LH-RH CAS 57773-63-4View Details
[D-Trp6]-LH-RH CAS 57773-63-4View Details
57773-63-4 -
 Avibactam sodium seriesView Details
Avibactam sodium seriesView Details
1192651-49-2 -
 Avibactam seriesView Details
Avibactam seriesView Details
1192651-80-1 -
 Avibactam Sodium SaltView Details
Avibactam Sodium SaltView Details
1192491-61-4 -
 Avibactam intermediateView Details
Avibactam intermediateView Details
1171080-45-7 -
 Relibatan intermediateView Details
Relibatan intermediateView Details
1416134-63-8
