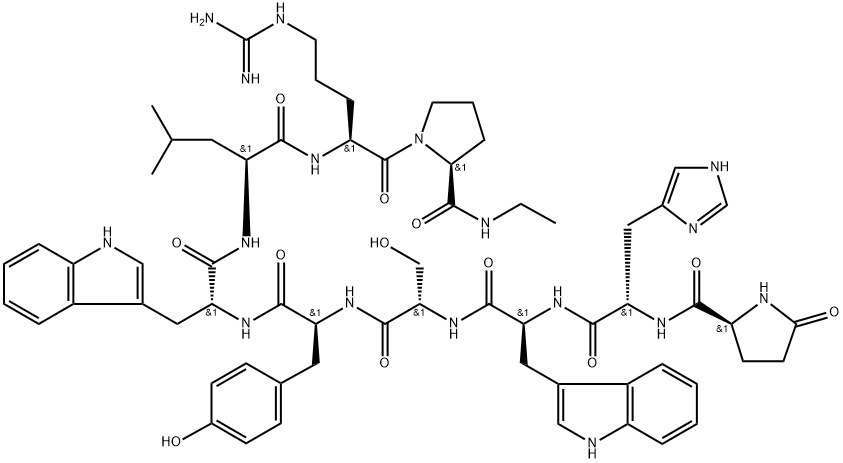
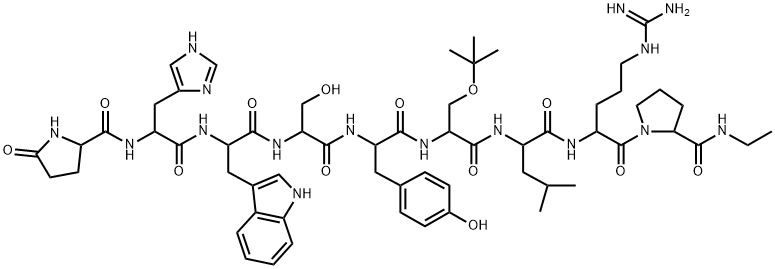
Abarelix
- CAS NO.:183552-38-7
- Empirical Formula: C72H95ClN14O14
- Molecular Weight: 1416.06
- MDL number: MFCD06407663
- EINECS: 1592732-453-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-24 14:33:46
What is Abarelix?
Absorption
Following IM administration of 100 mg, abarelix is absorbed slowly with a mean peak concentration of 43.4 ng/mL observed approximately 3 days after the injection.
Toxicity
The maximum tolerated dose of abarelix has not been determined. The maximum dose used in clinical studies was 150 mg. There have been no reports of accidental overdose with abarelix.
Description
Abarelix is an antagonist of the gonadotropin releasing-hormone (Gn RH) receptor, and it was launched as an intramuscular injection for the palliative treatment of advanced symptomatic prostate cancer. Hormonal therapy of prostate cancer is based on the modulation of testosterone to achieve medical castration levels. The inhibition of GnRH activity causes the suppression of luteinizing hormone (LH) and follicle stimulating hormone (FSH) secretion, thereby reducing the secretion of testosterone by the testes. Abarelix is the first GnRH antagonist to reach its market. Hormonal therapy with GnRH agonists such as leuprolide, buserelin, and goserelin has been in use for over two decades. Drugs of this type achieve the suppression of LH and FSH by a feedback inhibition mechanism, which involves an initial rise in the LH and FSH levels and, consequently, in the levels of testosterone. A testosterone surge may induce a clinical tumor flare that worsens cancer-related symptoms. This phenomenon is observed in 4–33% of patients receiving a GnRH agonist. A GnRH antagonist such as abarelix acts by direct inhibition of LH and FSH secretion, which avoids the initial surge in serum testosterone concentrations. Abarelix is a decapeptide, and it is prepared by a typical coupling cycle for peptide synthesis using Boc-amino acids and a methylbenzhydrylamine (MBHA) resin. Abarelix has high binding affinity for GnRH receptor (Kd=0.1 nM). Following intramuscular administration of a 100 mg dose, abarelix is absorbed slowly with a Cmax of 43.4 ng/mL observed approximately 3 days after the injection and has a half-life of about 13 days. The apparent volume of distribution is over 4000 L, suggesting extensive distribution. Abarelix has high protein binding (96–99%), and it is primarily metabolized via hydrolysis of peptide bonds. Following a dose of 15 μg/kg in humans, approximately 13% of abarelix is recovered unchanged in the urine, with no detectable metabolites. The renal clearance of abarelix is 14.4 L/day following a 100 mg dose. Two randomized, open label, comparative clinical trials involving 348 patients demonstrated the efficacy of abarelix versus a GnRH agonist (leuprolide) as well as a combination of GnRH agonist and anti-androgen (leuprolide+bicalutamide). In these trials, both abarelix and the comparators reduced testosterone to medical castration levels (<50 ng/dL) by day 29 of therapy in 94–98% of the patients. However, a significant difference was observed between the two groups for the occurrence of testosterone surge (0% in the abarelix group versus 82% in the GnRH agonist group) and for the rapidity of attaining castration levels (72% versus 0% on day 8 in the abarelix and GnRH agonist groups, respectively). Both groups maintained medical castration levels of testosterone with similar efficacy between days 29 and 85 of treatment. Abarelix was generally well tolerated in these trials. Approximately 3% of the patients experienced an immediate-onset allergic reaction. Other adverse events were similar to comparator controls and included hot flushes, sleep disturbance, pain, and breast enlargement. The recommended dosage of abarelix is 100 mg intramuscular injection on days 1, 15, and 29 of therapy, and every 4 weeks thereafter.
Originator
Praecis (US)
The Uses of Abarelix
Gonad-stimulating principle; antagonist (LHRH).
Indications
For palliative treatment of advanced prostate cancer.
What are the applications of Application
Abarelix Acetate is a synthetic peptide antagonist to GnRH receptors
Background
Synthetic decapeptide antagonist to gonadotropin releasing hormone (GnRH). It is marketed by Praecis Pharmaceuticals as Plenaxis. Praecis announced in June 2006 that it was voluntarily withdrawing the drug from the market.
Definition
ChEBI: A polypeptide compound composed of ten natural and non-natural amino acid resiudes in a linear sequence.
Manufacturing Process
Abbreviation Residue or moiety:
Nal - 3-(2-naphthyl)alaninyl
4-Cl-Phe - (4'-chlorophenyl)alaninyl
Pal - 3-(3'-pyridyl)alaninyl
Pal(N-O) - 3-(3'-pyridine-N-oxide)alaninyl
Pal(iPr) - 3-N-(2-propyl)-3'-pyridinium)alaninyl
BOC - N-t-butyoxycarbonyl
DCC - dicyclohexylcarbodiimide
Abarelix was synthesized by the solid phase method using an automated
synthesizer (e.g. Beckman Model 990). The amino acid residues used can be
purchased from commercial sources (e.g. Aldrich Chemical Co., Milwaukee,
Wis.), or can be produced from commercially available starting materials
according to known methods. Amino acids which are not obtained
commercially can be synthesized in a protected form for coupling, or, if
appropriate, can be coupled to form a peptide and subsequently modified to
the desired form.
For example, Boc-D-Pal(iPr) was prepared next way:
Boc-D-Pal (4.0 g, 17.7 mmol) and Ag2O (8.0 g, 34.4 mmol) in 22 ml water
was stirred at room temperature for 4 hours. The reaction vessel was cooled
to 0°C, and 2-iodopropane (20.4 g, 120 mmol) in 40 ml 2-propanol was
added. After addition was complete, the mixture was allowed to warm to room
temperature and stirred for 4 days. Additional Ag2O (2 g) and 2-iodopropane
(2 g) were added after 24 hours and again after 48 hours. The mixture was
filtered, and the precipitate was washed with ethanol (2x15 ml). The filtrate
was evaporated to yield 4.3 g of a yellow oil. Crystallization from
ethanol/ethyl acetate gave light yellow crystals (3.0 g); Yield: 63%; m.p.
182°-185°C.
Synthesis of Boc-D-Pal(N-O):
Boc-D-Pal (2.0 g, 7.5 mmole) was dissolved in 40 ml acetone and 2.48 g
(16.5 mmol) of m-chloroperbenzoic acid (MCPBA) (57-86%; purchased from
Aldrich and used as received) in 80 ml acetone was added in one portion. The
mixture was stirred at room temperature for 40 hours; a small amount of
white precipitate formed as the reaction proceeded. The precipitated was
filtered and the mother liquor evaporated to yield a white precipitate. The
combined solids were washed with ether (to remove chlorobenzoic acid) and
recrystallized from ethyl acetate/hexane. Yield: 1.7 g (80%); m.p. 155°-
157°C.
A typical coupling cycle for peptide synthesis with Boc-amino acids on a
peptide synthesizer (Beckman Model 990) was as follows:
Methylbenzyhydramine (MBHA) resin (1.18 g, 0.85 meq amino groups/g resin)
was weighed into the reaction vessel and washed with two portions of
chloroform (26 ml each). The resin was prewashed with 22% thioanisole (5
ml)/66% trifluoroacetic acid (TFA) in 14 ml dichloromethane (DCM) for 5
minutes, and then deprotected for 30 minutes with the same thioanisole/TFA
mixture. The resin was washed with three portions of chloroform (20 ml
each), two portions of 2-propanol (26 ml each) and two portions of DCM (26
ml each). The resin was neutralized with two portions of 12%
diisopropylethylamine (DIPEA) (26 ml each), and then washed with four
portions of DCM (26 ml each), followed by two portions of 1:1
DCM:dimethylformamide (DMF) (26 ml each). A solution of a Boc-protected
amino acid (2.5 mole equivalents) and 1-hydroxybenzotriazole (HOBt) (2.5
mole equivalents) was introduced as a solution in 10 ml DMF, and DCC was
added (256 mg in 6 DMF). Coupling was allowed to proceed for three hours,or overnight. Hindered residues (e.g. backbone N-methyl amino acids)required longer coupling times. The resin was washed with two 26 ml portions
of DMF, followed by two 26 ml portions of 2-propanol and then two 26 ml
portions of DCM. Completion of coupling was assessed by Kaiser's test
(ninhydrin test). If coupling is not complete, a double coupling was performed
(i.e. the resin was neutralized as above and the coupling step repeated).
When complete coupling is achieved, the cycle was repeated with the next
amino acid.
Upon completion of the synthesis, the peptide was cleaved from the resin by
treatment with liquid hydrofluoric acid (HF) for 45 minutes at 0°C. The HF was
evaporated and the peptide treated with aqueous acetic acid and lyophilized.
The crude peptide was then purified by high performance liquid
chromatography (HPLC) on a C18 column, eluting with a mixture of
acetonitrile and 0.1% TFA in water. Purified fractions (homogeneous by UV
and TLC analysis) were combined and lyophilized. Analytical HPLC was used to
determine the purity of the final product; peptides synthesized was at least
98% pure.
brand name
Plenaxis (Praecis).
Therapeutic Function
LHRH antagonist
Pharmacokinetics
Used in the palliative treatment of advanced prostate cancer. Abarelix is a luteinizing hormone agonist that results in suppression of testicular or follicular steroidogenesis.
Metabolism
In vitro hepatocyte (rat, monkey, human) studies and in vivo studies in rats and monkeys showed that the major metabolites of abarelix were formed via hydrolysis of peptide bonds. No significant oxidative or conjugated metabolites of abarelix were found either in vitro or in vivo. There is no evidence of cytochrome P-450 involvement in the metabolism of abarelix.
Properties of Abarelix
| Boiling point: | 1688.4±65.0 °C(Predicted) |
| Density | 1.286±0.06 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | Methanol (Slightly), Water (Slightly) |
| pka | 9.82±0.15(Predicted) |
| form | Solid |
| color | White to Off-White |
| CAS DataBase Reference | 183552-38-7 |
Safety information for Abarelix
Computed Descriptors for Abarelix
| InChIKey | AIWRTTMUVOZGPW-HSPKUQOVSA-N |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8