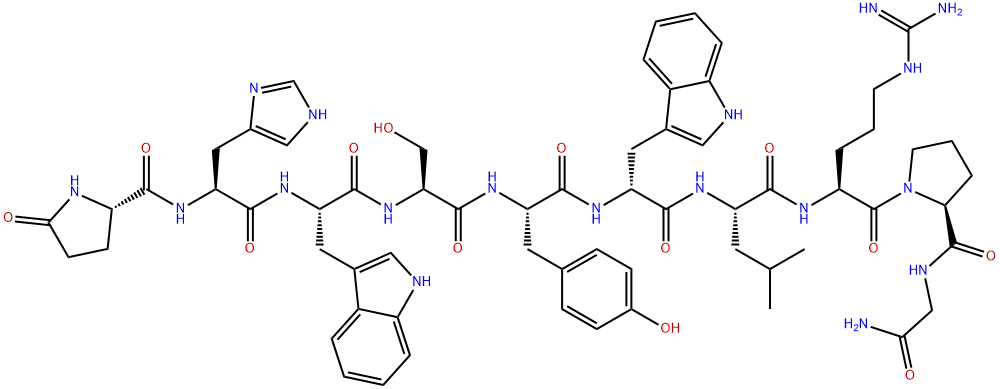
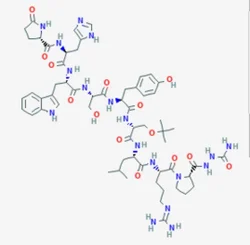
Leuprorelin
- CAS NO.:53714-56-0
- Empirical Formula: C59H84N16O12
- Molecular Weight: 1209.4
- MDL number: MFCD00167544
- EINECS: 633-395-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-29 22:33:22
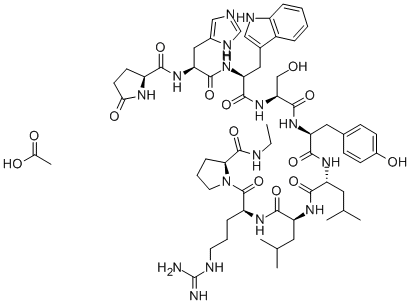
What is Leuprorelin?
Absorption
Leuprolide is typically administered as a single-dose long-acting formulation employing either microsphere or biodegradable solid depot technologies. Regardless of the exact formulation and initial dose strength, the Cmax is typically achieved by 4-5 hours post-injection and displays large variability in the range of 4.6 - 212 ng/mL. Eventual steady-state kinetics are typically achieved by four weeks, with a narrower range of 0.1 - 2 ng/mL. No studies on the effects of food on absorption have been carried out.
Toxicity
Leuprolide is considered extremely safe, with low dose-related toxicity and comparatively mild adverse effects. Prostate cancer patients treated with leuprolide at doses as high as 20 mg/day for two years showed no additional adverse effects compared to those receiving 1 mg/day.
Chemical properties
Hygroscopic, white or almost white powder.
Originator
Eligard,Atrix Laboratories, Inc.
The Uses of Leuprorelin
Gonadotropin releasing hormone (gonadorelin) analogue; treatment of prostate cancer.
The Uses of Leuprorelin
Highly active luteinizing hormone releasing hormone (LHRH) agonist
Indications
Leuprolide is indicated for the treatment of advanced prostate cancer and as palliative treatment of advanced prostate cancer.
It is also used for the treatment of pediatric patients with central precocious puberty (CPP).
In combination with oral norethisterone (also known as norethindrone), leuprolide is also indicated for the initial treatment of the symptoms of endometriosis. Finally, in combination with iron supplementation, leuprolide is indicated for the preoperative hematological improvement of anemic patients with uterine leiomyomata (uterine fibroids).
Background
Leuprolide is a synthetic 9-residue peptide analogue of gonadotropin-releasing hormone (GnRH). Unlike the endogenous decapeptide GnRH, leuprolide contains a single D-amino acid (D-leucyl) residue, which helps to increase its circulating half-life from three to four minutes to approximately three hours. As a GnRH mimic, leuprolide is capable of binding to the GnRH receptor (GnRHR) and inducing downstream modulation of both gonadotropin hormone and sex steroid levels. Prolonged activation of GnRHR results in significant downregulation of sex steroid levels, which is primarily responsible for the clinical efficacy of leuprolide in diverse conditions, including advanced prostate cancer, endometriosis, and central precocious puberty.
Leuprolide was first approved in 1985 as a daily subcutaneous injection under the tradename Lupron? by Abbvie Endocrine Inc. Since this initial approval, various long-acting intramuscular and subcutaneous products have been developed such that patients can be dosed once every six months. Leuprolide remains frontline therapy in all conditions for which it is indicated for use.
Definition
ChEBI: An oligopeptide comprising pyroglutamyl, histidyl, tryptophyl, seryl, tyrosyl, D-leucyl, leucyl, arginyl, and N-ethylprolinamide residues joined in sequence. Leuprorelin is a synthetic nonapeptide analogue of gonadotropin-releasi g hormone, and is used as a subcutaneous hydrogel implant (particularly as the acetate salt) for the treatment of prostate cancer and for the suppression of gonadal sex hormone production in children with central precocious puberty.
Indications
Leuprolide is a potent LH-RH agonist for the first several days to a few weeks after initiation of therapy, and therefore, it initially stimulates testicular and ovarian steroidogenesis. Because of this initial stimulation of testosterone production, it is recommended that patients with prostatic cancer be treated concurrently with leuprolide and the antiandrogen flutamide (discussed earlier). Leuprolide is generally well tolerated, with hot flashes being the most common side effect.
Therapeutic Function
Antineoplastic
Pharmacokinetics
Leuprolide is a gonadotropin-releasing hormone (GnRH) analogue that functions as a GnRH receptor superagonist. After an initial spike in GnRH-mediated steroidal production, including testosterone and estradiol, prolonged use results in a significant drop in circulating steroid levels, in line with those produced through other forms of androgen-deprivation therapy (ADT). The corresponding hormonal/steroidal changes produce specific adverse effects in different patient populations.
In women undergoing treatment for endometriosis or uterine leiomyomata, careful consideration regarding pregnancy status is advised. The initial increase in estradiol levels may worsen symptoms such as pain and bleeding. Long-term use of leuprolide is associated with loss of bone mineral density. Patients co-administered with norethisterone may experience sudden vision loss, proptosis, diplopia, migraine, thrombophlebitis, and pulmonary embolism and may also be at higher risk of cardiovascular disease. Patients with a history of depression may experience severe recurrence of depressive symptoms.
In men undergoing palliative treatment for advanced/metastatic prostate cancer, short-term spikes in testosterone levels may cause tumour flare and associated symptoms such as bone pain, hematuria, neuropathy, bladder and/or ureteral obstruction, and spinal cord compression. In addition, patients are at increased risk of developing hyperglycemia, diabetes, and cardiovascular disease, which may manifest through myocardial infarction, stroke, cardiac death, or prolonged QT/QTc interval. In addition, Leuprolide may cause convulsions and embryo-fetal toxicity.
In pediatric patients undergoing treatment for central precocious puberty (CPP), the initial steroidal spike may be associated with increased clinical signs of puberty within 2-4 weeks of treatment initiation. In addition, leuprolide may cause convulsions and psychiatric symptoms, including irritability, impatience, aggression, anger, and crying.
Synthesis
The synthesis process of Leuprorelin includes the following steps:
(1) Fmoc-Pro-HMPB-AM resin is obtained from Fmoc-Pro-OH and HMPB-AM resin with a substitution degree of 0.2mmol/g~1.2mmol/g as starting materials;
(2) The Fmoc-Pro-HMPB-AM resin was coupled one by one by Fmoc/tBu solid phase method to connect amino acids with protective groups in sequence, and the side chain fully protected leuprolide precursor peptide-HMPB-AM was synthesized Resin;
(3) Cut the side chain fully protected leuprolide precursor peptide-HMPB-AM resin to obtain the side chain fully protected leuprolide precursor peptide;
(4) Fully protected side chain leuprolide precursor peptide undergoes ethylamination to obtain side chain fully protected leuprolide;
(5) Leuprolide is fully protected on the side chain by removing the side chain protecting group to obtain the crude leuprolide peptide;
(6) The crude leuprorelin peptide is separated and purified by a high-pressure liquid phase column and lyophilized to obtain the leuprolide refined peptide.
Metabolism
Radiolabeling studies suggest that leuprolide is primarily metabolized to inactive penta-, tri-, and dipeptide entities, which are likely further metabolized. It is expected that various peptidases encountered throughout systemic circulation are responsible for leuprolide metabolism.
Properties of Leuprorelin
| alpha | D25 -31.7° (c = 1 in 1% acetic acid) |
| Density | 1.44±0.1 g/cm3(Predicted) |
| storage temp. | −20°C |
| solubility | DMSO (Slightly), Methanol (Slightly), Water (Slightly) |
| pka | 9.82±0.15(Predicted) |
| form | neat |
| Water Solubility | Soluble in water at 1mg/ml |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 53714-56-0(CAS DataBase Reference) |
Safety information for Leuprorelin
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H360:Reproductive toxicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Leuprorelin
| InChIKey | RGLRXNKKBLIBQS-VAZQWRJQNA-N |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Leuprolide 98%View Details
Leuprolide 98%View Details -
 53714-56-0 99%View Details
53714-56-0 99%View Details
53714-56-0 -
 Leuprolide 98%View Details
Leuprolide 98%View Details
53714-56-0 -
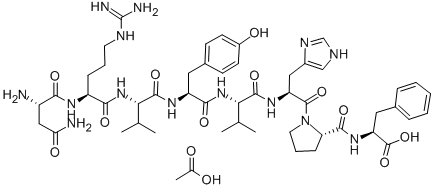
 Leuprorelin 99%View Details
Leuprorelin 99%View Details -
 Leuprorelin CAS 53714-56-0View Details
Leuprorelin CAS 53714-56-0View Details
53714-56-0 -
 LeuprorelinView Details
LeuprorelinView Details
74381-53-6 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
