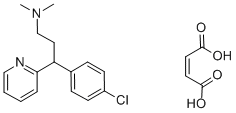
TRIMIPRAMINE MALEATE SALT
Synonym(s):Trimipramine maleate salt
- CAS NO.:521-78-8
- Empirical Formula: C24H30N2O4
- Molecular Weight: 410.51
- MDL number: MFCD00082376
- EINECS: 208-318-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-11 08:41:34
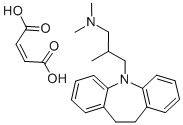
What is TRIMIPRAMINE MALEATE SALT?
Chemical properties
White Crystalline Solid
Originator
Surmontil Wyeth-Ayerst,Laboratories
The Uses of TRIMIPRAMINE MALEATE SALT
Antidepressant; serotonin transport blocker that also blocks norepinephrine uptake
The Uses of TRIMIPRAMINE MALEATE SALT
Antidepressant;Noradrenaline uptake inhibitor
The Uses of TRIMIPRAMINE MALEATE SALT
Antidepressant; serotonin transport blocker that also blocks norepinephrine uptake.
What are the applications of Application
Trimipramine Maleate Salt is a serotonin transport and norepinephrine uptake blocker
Manufacturing Process
Bis(3-dimethylamino-2-methylpropyl)-5-iminodibenzylyl carboxylic acid at 185-250°C up to discontinue a separation of oxyde carbonique. The product was dissolved in ether, then washed with the hydrochloric acid. Then this solution was extracted with ether. The solvent was evaporated under vacuum, to give pure oil bis(3-dimethylamino-2-methyl-1-propyl)-5-iminodibenzyle with boiling point 153-154°C at 0.4 mm. Maleate of bis(3-dimethylamino-2-methyl- 1-propyl)-5-iminodibenzyle have melting point 145-146°C.
brand name
Surmontil (Odyssey).
Therapeutic Function
Antidepressant
General Description
For details of chemical nomenclature, consult the descriptionof imipramine. Replacement of hydrogen with an -methylsubstituent produces a chiral carbon, and trimipramine(Surmontil) is used as the racemic mixture. Biological propertiesreportedly resemble those of imipramine.
Biological Activity
trimipramine (maleate) is a potent antagonist of histamine h1 receptor, serotonin 5-ht2a, and α1-adrenergic receptors [1].the histamine h1 receptor is widely expressed tissues, such as smooth muscles, vascular endothelial cells, heart, and the central nervous system. histamine h1 receptor (h1r) antagonists are very effective drugs alleviating the symptoms of allergic reactions [2]. 5-ht2a receptor shows constitutive activity. variations in5-ht2a receptor can produce profound alterations in cognitive states [3]. the adrenergic receptors play an important role in modulating sympathetic nervous system activity as well as a site of action for many therapeutic agents. the α1-adrenergic receptor is the prime mediators of smooth muscle contraction and hypertrophic growth. the α1-adrenergic receptor plays an essential role in smooth muscle, growth, neurological, and cardiovascular function [4].trimipramine (maleate) is a tricyclic antidepressant compound that antagonizes several types of neurotransmitter receptors. trimipramine potently antagonized the activity of histamine h1 serotonin 5-ht2a, and α1-adrenergic receptors with the kd value of 0.27 nm, 24 nm, and 24 nm, respectively. trimipramine moderately antagonized the activity of dopamine d2 with the kd of 180 nm. trimipramine inhibited the activity of muscarinic acetylcholine with the kd of 58 nm. trimipramine weakly inhibited the activity of 5-ht2c, d1, α2-adrenergic receptors (kd = 680 nm) [1]. trimipramine (maleate) was a weak to moderate reuptake inhibitor of serotonin (ki = 149 nm for sert), and an extremely weak inhibitor of norepinephrine (ki = 2.5 μm for net) and dopamine (ki = 3.8 μm for dat) reuptake in slices of rat cerebral cortex [5,6].
Biochem/physiol Actions
Antidepressant; serotonin transport blocker that also blocks norepinephrine uptake.
References
[1] richelson e, nelson a. antagonism by antidepressants of neurotransmitter receptors of normal human brain in vitro[j]. journal of pharmacology and experimental therapeutics, 1984, 230(1): 94-102.
[2] shimamura t, shiroishi m, weyand s, et al. structure of the human histamine h1 receptor complex with doxepin[j]. nature, 2011, 475(7354): 65-70.
[3] harvey j a. role of the serotonin 5-ht2a receptor in learning[j]. learning & memory, 2003, 10(5): 355-362.
[4] piascik m t, perez d m. α1-adrenergic receptors: new insights and directions[j]. journal of pharmacology and experimental therapeutics, 2001, 298(2): 403-410.
[5] gross g, xie x, gastpar m. trimipramine: pharmacological reevaluation and comparison with clozapine[j]. neuropharmacology, 1991, 30(11): 1159-1166.
[6] tatsumi m, groshan k, blakely r d, et al. pharmacological profile of antidepressants and related compounds at human monoamine transporters[j]. european journal of pharmacology, 1997, 340(2): 249-258.
Properties of TRIMIPRAMINE MALEATE SALT
| Melting point: | 141-143°C |
| Flash point: | 11 °C |
| storage temp. | 2-8°C |
| solubility | chloroform: soluble50 mg/ml, clear, colorless to faintly yellow |
| form | powder |
| color | white |
| EPA Substance Registry System | Trimipramine maleate (521-78-8) |
Safety information for TRIMIPRAMINE MALEATE SALT
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H361:Reproductive toxicity |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for TRIMIPRAMINE MALEATE SALT
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran



You may like
-
 521-78-8 Trimipramine maleate 98%View Details
521-78-8 Trimipramine maleate 98%View Details
521-78-8 -
 521-78-8 99%View Details
521-78-8 99%View Details
521-78-8 -
 Trimipramine maleate 98%View Details
Trimipramine maleate 98%View Details
521-78-8 -
 Trimipramine Maleate CAS 521-78-8View Details
Trimipramine Maleate CAS 521-78-8View Details
521-78-8 -
 Trimipramine maleate CAS 521-78-8View Details
Trimipramine maleate CAS 521-78-8View Details
521-78-8 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
