Triclopyr
Synonym(s):3,5,6-Trichloro-2-pyridinyloxyacetic acid
- CAS NO.:55335-06-3
- Empirical Formula: C7H4Cl3NO3
- Molecular Weight: 256.47
- MDL number: MFCD00072514
- EINECS: 259-597-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
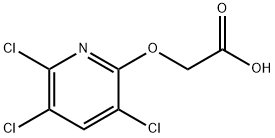
What is Triclopyr?
Description
Triclopyr is a trichloropyridine-based broadleaf herbicide that was developed by Dow Chemical (Midland, MI) in the 1970s. It was originally synthesized in 1958 by M. P. Cava and N. K. Bhattacharyya at Ohio State University (Columbus).
Triclopyr is a mimic of a plant growth hormone called an auxin. This type of herbicide kills the target weed by imitating the auxin indole-3-acetic acid1. When it is administered at effective doses, it causes uncontrolled, disorganized plant growth that leads to plant death.
Despite its three chlorine atoms, triclopyr is a relatively safe, nonpersistent pesticide. It was originally developed as a crop-protection product, but it and other herbicides have more recently been used to combat invasive plant species.
One example is triclopyr’s use against the Hawaiian invasion of miconia trees (Miconia calvescens) from Central and South America. Miconia grows and spreads rapidly; and its large leaves block the sunlight from native plants. To make matters worse, birds are fond of miconia fruit and spread the plant’s seeds, often in inaccessible locations.
The Big Island (Hawai‘i) is already overrun with miconia, but the trees have made only small inroads into the nearest island, Maui. Scientists James Leary at the University of Florida (Gainesville) and Adam Knox of the Maui Invasive Species Committee of the University of Hawai‘i developed and use an innovative technique to locate and eradicate the plants.
Leary, while at the University of Hawai‘i at Manoa about 15 years ago, prepared a triclopyr formulation that can be used to make paintball capsules. For the past decade, he, Knox, and their team have used helicopters to locate and “shoot” miconia with herbicidal paintballs. During that period, they have killed ≈26,000 of the invasive species.
1. CAS Reg. No. 87-51-4.
Chemical properties
White to Off-White Solid
The Uses of Triclopyr
Triclopyr is a selective post-emergence herbicide.
The Uses of Triclopyr
Selective postemergence herbicide to control many woody and broad-leaved weeds.
Definition
ChEBI: A monocarboxylic acid that is (pyridin-2-yloxy)acetic acid substituted by chloro groups at positions 3, 5 and 6. It is an agrochemical used as a herbicide.
Agricultural Uses
Herbicide: Triclopry is a systemic herbicide used on rice, range land and pasture, rights-of-way, forestry and grasslands, including home lawns, for control of broadleaf weeds and woody plants. Triclopry is usually available as a triclopyr butoxyethyl ester (BEE) or as a triclopry triethylamine salt (TEA). Registered for use in the U.S. Some products may be classified as Restricted Use Pesticides (RUP). Registered for use in EU countries . U.S. Maximum Allowable Residue Levels for Triclopyr and its metabolites 3,5,6,-trichloro-2-pyridinol and 2-methoxy-3,5,6-trichloropyridine [40 CFR 180.417 (a)(1)]: for the combined residues of the herbicide in or on the following raw agricultural commodities: fish 3.0 ppm; grass, forage 500 ppm; grass, forage, hay 500 ppm;shellfish3.5 ppm.[40 CFR 180.417 (a)(2)]: in or on the following agricultural commodities: egg 0.05 ppm; meat, fat, and meat byproducts, except kidney and liver, of cattle, goat, hog, horse and sheep 0.05 ppm; meat, fat, and meat byproducts, except kidney, of poultry 0.1 ppm; milk 0.01 ppm; liver and kidney of cattle, goat, hog, horse and sheep 0.5 ppm; rice, grain 0.3 ppm; rice, straw10.0 ppm.
Trade name
[Note: See the following record, Triclopyr, triethylamine salt, for trade names containing the salt of triclopry] ACCESS®; CROSSBOW®, (triclopyr + 2,4-D ester); ET®; GARLON®; GRAZON® Triclopyr; PATHFINDER®; REDEEM®; RELY®; REMEDY®; RIVERDALE TAHOE® Nufarm (Australia); TURFLON®
Safety Profile
Moderately toxic by ingestion. Anexperimental teratogen. Experimental reproductiveeffects. Used as an herbicide. When heated todecomposition it emits toxic fumes of Clí and NOx.
Environmental Fate
Soil. In soil, triclopyr degraded to 3,5,6-trichloro-2-pyridinol and 2-methoxy-3,5,6-
trichloropyridine (Norris et al., 1987). The major route of dissipation from soil is likely
due to microbial degradation (Newton et al., 1990).
Fifty-four days after triclopyr was applied to soil at a rate of 5.6 kg/ha, the majority
(65%) remained in the top 10 cm of soil (Lee et al., 1986a).
Ashton and Monaco (1991) reported triclopyr had an average half-life of 46 days and
is in?uenced by soil type and climatic conditions.
Plant. Lewer and Owen (1989) found 3,5,6-trichloro-2-pyridinol as the major metab-
olite in plants. Cultured soybean cells metabolized triclopyr to dimethyl triclopyr-aspartate
and dimethyl triclopyr-glutamate which can be rehydrolyzed to form the parent compound.
At an application rate of 3.4 kg/ha, the dissipation half-life was 3.7 days. At an
application rate of 3.3 kg/ka, the dissipation half-lives of triclopyr in brown, browse and
litter samples were 73.5, 202.3 and 31 days, respectively (Norris et al., 1987).
Metabolic pathway
After 7 days in a soybean cell suspension culture, the major metabolites of trichlopyr are formed and identified as the aspartate (major) and glutamate (minor) amide conjugates.
Metabolism
The average half-life of triclopyr in soil is 46 days. Because triclopyr is sensitive to light, the length of time that it remains on the surface may moderate its persistence. Adsorption to soil is limited and is governed by the anion exchange capacity. Insignificant amounts are lost via volatilization. The propensity of triclopyr to translocate in the environment with soil water, is unknown.
Toxicity evaluation
The acute oral LD50 of triclopyr is 692 mg/kg and 577 mg/kg for male and female rat, respectively. It does not appear to undergo significant transformation and is excreted in the urine primarily unchanged.
Properties of Triclopyr
| Melting point: | 148-150°C |
| Boiling point: | 290°C |
| Density | 1.7626 (rough estimate) |
| refractive index | 1.6200 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Chloroform (Slightly), Ethyl Acetate (Slightly), Methanol (Slightly) |
| appearance | fluffy colorless or white crystals or solid |
| form | Liquid |
| pka | 2.68(at 25℃) |
| color | Fluffy solid |
| Water Solubility | 0.43g/L(temperature not stated) |
| Merck | 13,9730 |
| BRN | 225301 |
| CAS DataBase Reference | 55335-06-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Acetic acid, [(3,5,6-trichloro-2-pyridinyl)oxy]-(55335-06-3) |
| EPA Substance Registry System | Triclopyr (55335-06-3) |
Safety information for Triclopyr
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H319:Serious eye damage/eye irritation H371:Specific target organ toxicity, single exposure H373:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P391:Collect spillage. Hazardous to the aquatic environment P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. P405:Store locked up. |
Computed Descriptors for Triclopyr
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran


You may like
-
 55335-06-3 98%View Details
55335-06-3 98%View Details
55335-06-3 -
 Triclopyr CAS 55335-06-3View Details
Triclopyr CAS 55335-06-3View Details
55335-06-3 -
 Triclopyr 98% CAS 55335-06-3View Details
Triclopyr 98% CAS 55335-06-3View Details
55335-06-3 -
 Triclopyr CAS 55335-06-3View Details
Triclopyr CAS 55335-06-3View Details
55335-06-3 -
 Triclopyr CAS 55335-06-3View Details
Triclopyr CAS 55335-06-3View Details
55335-06-3 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 609-15-4View Details
609-15-4View Details
609-15-4 -
 27143-07-3View Details
27143-07-3View Details
27143-07-3
