Tribenuron methyl
- CAS NO.:101200-48-0
- Empirical Formula: C15H17N5O6S
- Molecular Weight: 395.39
- MDL number: MFCD03955329
- EINECS: 401-190-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-04-29 17:45:46
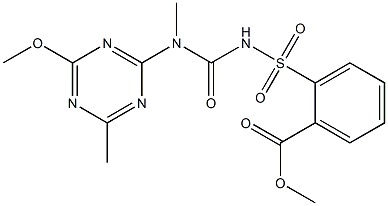
What is Tribenuron methyl?
Chemical properties
Tribenuron-methyl (TBM) is a member of the sulfonylurea herbicide family. It is a relatively polar, weak, acidic compound that is soluble in water. It can be extracted with polar organic solvent, buffer solution, or a mixture of organic solvent and water.
Tribenuron-methyl is a selective herbicide that inhibits the biosynthesis of branched amino acids in sensitive plants by competitively binding to the enzyme system which catalyzes the formation of these amino acids, the acetolactate synthase (ALS). It is used in cereals for the control of broadleaved weeds.
The Uses of Tribenuron methyl
Tribenuron-methyl is a sulfonyl urea herbicide derivative employed for protection of crops such as corn, citrus, potatoes, tomatoes, rice and vines via inhibiting the growth of many grasses and broad-leaf weed species.Tribenuron-methyl is applied as a foliar spray and directly to the soil as it is rapidly absorbed by the foliage and the roots, and is translocated throughout the plant to the growing points.
Tribeneuron-methyl acts by inhibiting the synthesis of amino acids, specifically valine and isoleucine, which prevents cell division and cell growth. Selectivity occurs due to the differences in the metabolism of sulfonylureas between crops and weeds.
Methomyl is a broad spectrum carbamate insecticide with both contact and oral toxicity to control chewing and sucking Lepidotera, Homoptera, Coleoptera and Hemiptera pests in vegetable, orchard, vine and field crops.
Definition
ChEBI: The methyl ester of tribenuron.
General Description
Colorless crystals. Non corrosive. Used as an herbicide.
Air & Water Reactions
Hydrolysis occurs rapidly at pH <7 or >12.
Reactivity Profile
A sulfonylurea derivative.
Agricultural Uses
Herbicide: Used to control annual grasses and broadleaf weeds on barley, blueberries, oats, wheat, flax and rape seed (canola). Registered for use in EU countries . Registered for use in the U.S. U.S. Maximum Allowable Residue Levels for the residues of the herbicide tribenuron methyl [40 CFR 180.451(a)]: in or on the following raw agricultural commodities: barley, grain 0.05 ppm; barley, straw 0.10ppm; canola, seed 0.02 ppm; cotton, gin byproducts 0.02 ppm; cotton, undelinted seed 0.02 ppm; flax, seed 0.02 ppm; oat, grain 0.05 ppm; oat, straw 0.10ppm; wheat, grain 0.05ppm; and wheat, straw 0.10 ppm. Tolerances with regional registration, as defined in 180.1(n), are established. [40 CFR 180.451(c)]: in or on the following raw agricultural commodities: grass, forage, fodder and hay, group (except Bermudagrass); forage 0.10ppm; and grass, forage, fodder and hay, group (except Bermudagrass); hay 0.10 ppm.
Trade name
ALLY®; CANVAS®; DPX-L-5300®; EXPRESS®; EXPRESS®-75 DF; HARMONY EXTRA®; INL-5300®; L 5300®; MATRIX®
Metabolic pathway
The chemical structure of tribenuron methyl possesses a mono-N-methyl group in the sulfonylurea linkage which is a unique sulfonylurea herbicide and undergoes complex degradation reactions in the environment. Tribenuron methyl degrades into many degradation products, as indicated in the pathways by photolytic and hydrolytic chemical reactions and by biological degradations by mammal, plant, and soil. It is interesting to note that photolysis in different solvents yields diverse products depending on the individual solvents.
Degradation
Methomyl (1) was hydrolysed under alkaline conditions at 25 °C (DT50
ca. 14 days) and was stable under neutral to acidic conditions [DT50 > 30
days at pH 5 and 7 (Friedman, 1983)l. Cleavage of the carbamate ester
bond is the primary degradation pathway, yielding S-methyl N-hydroxy-thioacetimidate
(2).
Irradiation of methomyl in dilute aqueous solution at 254 nrn yielded
acetonitrile (3), dimethyl disulfide, acetone, N-ethylidenethylamine and
carbon dioxide (Freeman and Ndip, 1984). Methomyl does not absorb
sunlight but underwent degradation to acetonitrile by indirect photolytic
reactions in/on soil surfaces (Swanson, 1986).
Properties of Tribenuron methyl
| Melting point: | 141°C |
| Density | 1.4143 (rough estimate) |
| vapor pressure | 1.24 at pH 7 (25 °C) |
| refractive index | 1.6460 (estimate) |
| storage temp. | 0-6°C |
| solubility | Chloroform (Slightly), DMSO (Slightly) |
| pka | 4.34±0.10(Predicted) |
| form | neat |
| Water Solubility | 55 g l-1(25 °C) |
| Stability: | Hygroscopic, Unstable in Solution |
| CAS DataBase Reference | 101200-48-0(CAS DataBase Reference) |
| EPA Substance Registry System | Tribenuron-methyl (101200-48-0) |
Safety information for Tribenuron methyl
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H373:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P272:Contaminated work clothing should not be allowed out of the workplace. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P314:Get medical advice/attention if you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for Tribenuron methyl
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran





You may like
-
 Tribenuron-methyl CAS 101200-48-0View Details
Tribenuron-methyl CAS 101200-48-0View Details
101200-48-0 -
 Matrix 256GB X-Stream Edition M.2 SSDView Details
Matrix 256GB X-Stream Edition M.2 SSDView Details
101200-48-0 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
