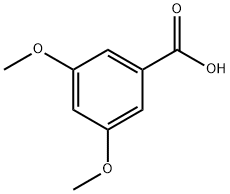
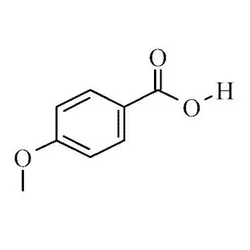
4-Methoxybenzoic acid
Synonym(s):p-Anisic acid;p-Methoxybenzoic acid;4-Methoxybenzoic acid;Draconic acid;p-Anisic acid
- CAS NO.:100-09-4
- Empirical Formula: C8H8O3
- Molecular Weight: 152.15
- MDL number: MFCD00002542
- EINECS: 202-818-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:59
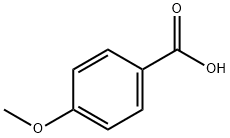
What is 4-Methoxybenzoic acid?
Description
4-methoxybenzoic acid, also known as p-Anisic acid or draconic acid, is an organic acid with a sweet flavor. It is one of the isomers(m-anisic acid, and o-anisic acid) of anisic acid. The term "anisic acid" often refers to this form specifically. P-anisic acid is produced through the oxidation of p-cresyl-methyl ether.
Chemical properties
It appears as a colorless needle crystal at room temperature. It is soluble in ethanol, ether, and chloroform, and only slightly soluble in hot water. It remains insoluble in cold water. It is used as an intermediate for aniracetam, as well as a preservative and fragrance.
The Uses of 4-Methoxybenzoic acid
4-Methoxybenzoic acid (p-Anisic acid) is used in oxidation and reduction of cytochrome c in solution through different self-assembled monolayers on gold electrodes using cyclic voltammetry. p-Anisic acid has antiseptic properties. It is also used as an intermediate in the preparation of more complex organic compounds.
Definition
ChEBI: 4-methoxybenzoic acid is a methoxybenzoic acid substituted with a methoxy group at position C-4. It has a role as a plant metabolite. It is functionally related to a benzoic acid. It is a conjugate acid of a 4-methoxybenzoate.
Preparation
To obtain 4-Methoxybenzoic acid, the mole ratio of cobalt and manganese is modified and p-methoxy toluene is reacted with oxygen or an oxygen-containing gas in the presence of acetic acid. The following is the experimental procedure:1. Using n-hexyl bromide, tri (n-hexyl) amine, para-methoxy toluene with cobalt chloride hexahydrate in about 9h;
2. By a catalytic oxidation process using p-methoxy toluene and propionic acid over a catalyst comprising of CoBr2.6H2O and MnBr2.4H2O with a reaction time of 20h;
3. Through changing the mole ratio of cobalt and manganese, using p-methoxy toluene with oxygen or oxygen containing gas in the presence of acetic acid to obtain the product.
Synthesis Reference(s)
Journal of Heterocyclic Chemistry, 25, p. 973, 1988 DOI: 10.1002/jhet.5570250351
Tetrahedron Letters, 34, p. 4603, 1993 DOI: 10.1016/S0040-4039(00)60635-4
General Description
4-Methoxybenzoic acid is the sole source of carbon and energy for growth in the cultures of Nocardia sp. DSM 1069. It is effective in clearing congestion in the lungs and the respiratory tracts in conditions like asthma or bronchitis.
Reactivity Profile
4-Methoxybenzoic acid belongs to Aromatic acid hydrazides (Others include isonicotinic, benzoic, and salicylic). The reaction of thiosemicarbazide and aromatic acid hydrazides with 4H-chromen-4-imines could prepare o-hydroxyphenyl derivatives of 1H-pyrazolethioamides and 1H-pyrazol-5-ylphenols derivatives[4].
Hazard
Thermal decomposition can lead to release of irritating gases and vapors, Carbon monoxide (CO), Carbon dioxide (CO2).
Biochem/physiol Actions
The cytochrome P450 enzyme CYP199A4, a heme-dependent cytochrome P450 (CYP) monooxygenase enzyme, can efficiently demethylate 4-methoxybenzoic acid. Mechanistically, O-demethylation of 4-methoxybenzoic acid occurs via hydrogen abstraction by Compound I, followed by oxygen rebound to give the hydroxylated hemiacetal which spontaneously decomposes to yield 4-hydroxybenzoic acid and formaldehyde[5].
Safety Profile
Poison by subcutaneous route.When heated to decomposition it emits acrid smoke andirritating vapors.
Applications
4-Methoxybenzoic acid was used in oxidation and reduction of cytochrome c in solution through different self-assembled monolayers on gold electrodes using cyclic voltammetry. It is also the sole source of carbon and energy for growth in the cultures of Nocardia sp. DSM 1069.
Solubility in organics
Soluble in alcohol and oils.
Purification Methods
Crystallise p-anisic acid from EtOH, water, EtOH/water or toluene. The S-benzylisothiuronium salt has m 189o (from EtOH). [Beilstein 10 II 91, 10 III 280, 10 IV 346.]
References
[1] Michael Ash (2004) Handbook of Preservatives
[2] Asim Kumar Mukhopadhyay (2004) Industrial Chemical Cresols and Downstream Derivatives
[3] https://en.wikipedia.org/wiki/P-Anisic_acid
[4] Zahorulko S, et al. Recyclization of 4 H -chromen-4-imine derivatives under the influence of dinucleophiles with the formation of functionally substituted pyrazoles. Monatshefte für Chemie - Chemical Monthly, 2019; 150: 1487–1493.
[5] Coleman T, et al. The importance of the benzoic acid carboxylate moiety for substrate recognition by CYP199A4 from Rhodopseudomonas palustris HaA2. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics, 2016; 1864: 667-675.
Properties of 4-Methoxybenzoic acid
| Melting point: | 182-185 °C(lit.) |
| Boiling point: | 275 °C |
| Density | 1.385 |
| vapor pressure | 0.001Pa at 25℃ |
| FEMA | 3945 | 4-METHOXYBENZOIC ACID |
| refractive index | 1.571-1.576 |
| Flash point: | 185 °C |
| storage temp. | Store below +30°C. |
| solubility | H2O: soluble2500 parts |
| form | Powder |
| pka | 4.50(at 25℃) |
| color | White to slightly gray-beige |
| PH | 3-4 (0.3g/l, H2O, 20℃) |
| Odor | at 10.00 % in dipropylene glycol. faint putrid sweet cadaverous |
| Water Solubility | Soluble in water at 20°C 0.3g/L. Soluble in alcohol, ethyl acetate and ether. |
| Merck | 14,666 |
| JECFA Number | 883 |
| BRN | 508910 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 100-09-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzoic acid, 4-methoxy-(100-09-4) |
| EPA Substance Registry System | Benzoic acid, 4-methoxy- (100-09-4) |
Safety information for 4-Methoxybenzoic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 4-Methoxybenzoic acid
| InChIKey | ZEYHEAKUIGZSGI-UHFFFAOYSA-N |
4-Methoxybenzoic acid manufacturer
JSK Chemicals
SAKEM LLP
Aagam Global Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 100 - 09 - 4 4 METHOXY BENZOIC ACID/ PARA ANISC ACID 99%View Details
100 - 09 - 4 4 METHOXY BENZOIC ACID/ PARA ANISC ACID 99%View Details
100 - 09 - 4 -
 p-Anisic acid 98%View Details
p-Anisic acid 98%View Details -
 p-Anisic acid 98%View Details
p-Anisic acid 98%View Details -
 Para Anisic Acid CASView Details
Para Anisic Acid CASView Details -
 p-Anisic acid CASView Details
p-Anisic acid CASView Details -
 p-Anisic Acid CAS 100-09-4View Details
p-Anisic Acid CAS 100-09-4View Details
100-09-4 -
 Powder 4-Methoxybenzoic Acid,P-Methoxybenzoic AciView Details
Powder 4-Methoxybenzoic Acid,P-Methoxybenzoic AciView Details
100-09-4 -
 White Powder Para Anisic Acid CAS No.100-09-4, Packaging Type: Cardboard Drum, Packaging Size: 25 kgView Details
White Powder Para Anisic Acid CAS No.100-09-4, Packaging Type: Cardboard Drum, Packaging Size: 25 kgView Details
100-09-4
