Tetramethylbenzidine
Synonym(s):TMB;3,3′,5,5′-Tetramethylbenzidine;TMB substrate;3,3?,5,5?-Tetramethylbenzidine;TMB, Insoluble
- CAS NO.:54827-17-7
- Empirical Formula: C16H20N2
- Molecular Weight: 240.34
- MDL number: MFCD00007748
- EINECS: 259-364-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-10-27 17:41:02
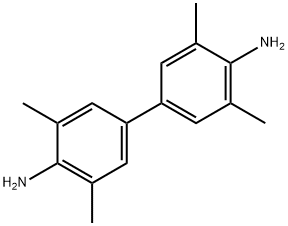
What is Tetramethylbenzidine?
Description
Tetramethylbenzidine(TMB) is a chromogenic substrate used in staining procedures in immunohistochemistry as well as being a visualising reagent used in enzyme-linked immunosorbent assays (ELISA). It is a white solid that forms a pale blue-green liquid in solution with ethyl acetate. TMB is degraded by sunlight and by fluorescent lights. It is a soluble chromogen substrate for horseradish peroxidase detection systems. Tetramethylbenzidine is recommended for ELISA procedures.
Chemical properties
Tetramethylbenzidine is a white or light yellow solid, odorless, tasteless, insoluble in water, easily soluble in acetone, ether, dimethyl sulfoxide, dimethylformamide and other organic solvents. It forms a pale blue-green liquid in solution with ethyl acetate. TMB is degraded by sunlight and by fluorescent lights.
The Uses of Tetramethylbenzidine
3,3′,5,5′-Tetramethylbenzidine may be used as a substrate for the analysis of diclofenac in water samples and dextromethorphan and its major metabolite dextrorphan in urine samples using enzyme-linked immunosorbent assay (ELISA) and gas chromatography coupled to mass spectrometry (GC-MS).
The Uses of Tetramethylbenzidine
3,3,5,5-Tetramethyl benzidine is used as a reagent in a sensitive staining procedure for the detection of low levels of heme-associated peroxidase activity of cytochrome P-450 on SDS-polyacrylamide or agarose gel; non-carcinogenic substitute for benzidine as reagent for the detection of blood and determination of hemoglobin content.
What are the applications of Application
3,3′,5,5′-Tetramethylbenzidine is a sensitive reagent for the detection of hemoglobin
Preparation
The synthesis of 3,3',5,5'-Tetramethylbenzidine involved using 2,6-dimethylaniline as the raw material. The process included activation, oxidative coupling, and purification, resulting in the production of pure material. The yield of 3,3,5,5-tetramethylbenzidine was 65%.
Reactions
TMB can act as a hydrogen donor for the reduction of hydrogen peroxide to water by peroxidase enzymes such as horseradish peroxidase.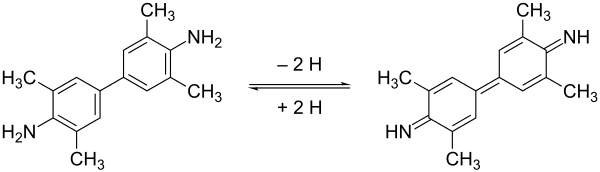
Shows the oxidation of 3,3′,5,5′-tetramethylbenzidine (TMB) to 3,3',5,5'-tetramethylbenzidine diimine
The resulting diimine causes the solution to take on a blue colour, and this colour change can be read on a spectrophotometer at the wavelengths of 370 and 650 nm.
General Description
3,3',5,5'-tetramethylbenzidine appears as pale yellow crystals or off-white powder. (NTP, 1992)
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Tetramethylbenzidine is sensitive to prolonged exposure to light . Neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides.
Fire Hazard
Flash point data for Tetramethylbenzidine are not available, however, Tetramethylbenzidine is probably combustible.
Biochem/physiol Actions
3,3′,5,5′-Tetramethylbenzidine/TMB can be used as a chromogen to increase the progression of product obtained in peroxidase reaction. In food and environmental decontamination procedures, TMB can be used in in?situ free available chlorine (FAC) monitoring of chlorite-based sanitizers.
Carcinogenicity
The carcinogenic potential of Tetramethylbenzidine is uncertain due to conflicting evidence. It is not mutagenic by the Ames test, and did not induce formation of tumors in a single-arm study of 24 rats. On that evidence, it has been used as a replacement for carcinogenic compounds such as benzidine and o-phenylenediamine.
DOI: 10.1177/26.2.24068
Properties of Tetramethylbenzidine
| Melting point: | 168-171 °C(lit.) |
| Boiling point: | 100 °C |
| Density | 1 |
| refractive index | 1.5519 (estimate) |
| storage temp. | 2-8°C |
| solubility | Slightly soluble. <0.1 g/100 mL at 20°C. |
| form | tablet |
| pka | 4.49±0.10(Predicted) |
| color | Cream |
| Water Solubility | Slightly soluble. <0.1 g/100 mL at 20 ºC |
| Sensitive | Light Sensitive |
| BRN | 2808541 |
| Stability: | Stable, but moisture sensitive and may be light sensitive. Incompatible with water, strong oxidizing agents. |
| CAS DataBase Reference | 54827-17-7(CAS DataBase Reference) |
| EPA Substance Registry System | 3,3',5,5'-Tetramethylbenzidine (54827-17-7) |
Safety information for Tetramethylbenzidine
| Signal word | Warning |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H302:Acute toxicity,oral H312:Acute toxicity,dermal H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H332:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H370:Specific target organ toxicity, single exposure |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Tetramethylbenzidine
Tetramethylbenzidine manufacturer
Suvchem
New Products
BOC-L-4-HYDROXYPROLINE 2-nitro 3-hydroxy pyridine 2,6-Dichloropyridin-4-amine 2,3 Diamino pyridine 5-Iodo-2-(1-methylethyl)-3(2H)-pyridazinone 1-Azetidinecarboxylic acid, 3-[(3S)-1-(trans-3-carboxy-3-methylcyclobutyl)-3-piperidinyl]-, 1-(1,1-dimethylethyl) ester Tert-Butyl N-[3-(dimethylcarbamoyl)prop-2-en-1-yl]carbamate 1-(difluoromethyl)-N-methylcyclobutan-1-amine 2-(4-Methyl-1,2,5-oxadiazol-3-yl)-1H-benzimidazole 6-(4-iodophenyl)-1-oxa-6-azaspiro[3.3]he Trimethyl(phenylthio)silane Polycaprolactone(2000)-PEG(20000)-Polycaprolactone(2000) Diacrylate Diethylene Glycol Monoethyl Ether, PolyoxyethyleneOleylCetylEtherSulfosuccinate Ascorbyl Tetraisopalmitate or Tetrahexyldecyl Ascorbate Castor Oil, Ethoxylated, Cremophor EL or PEG-35 Castor Oil Tween 20 or Polysorbate 20 Acetone-d6 (R)-2-Mercaptobutanoic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine 3-(naphthalen-1-ylsulfonyl)-1H-indazol-5-amine methyl 5-amino-3-(1,1-dioxidotetrahydro-2H-1,2-thiazin-2-yl)-2-fluorobenzoate 7-methoxy-8-(2-morpholinoethoxy)-4-((3,4,5-trimethoxyphenyl)amino)benzo[g]quinoline-3-carbonitrile Dimethylaluminum isopropoxideRelated products of tetrahydrofuran

You may like
-
 3,3,5,5-Tetramethylbenzidine (TMB) extrapure AR CAS 54827-17-7View Details
3,3,5,5-Tetramethylbenzidine (TMB) extrapure AR CAS 54827-17-7View Details
54827-17-7 -
![3,3',5,5'-Tetramethylbenzidine [for Biochemical Research] CAS 54827-17-7](https://img.chemicalbook.in//Content/image/CP5.jpg) 3,3',5,5'-Tetramethylbenzidine [for Biochemical Research] CAS 54827-17-7View Details
3,3',5,5'-Tetramethylbenzidine [for Biochemical Research] CAS 54827-17-7View Details
54827-17-7 -
 3,3′,5,5′-Tetramethylbenzidine CAS 54827-17-7View Details
3,3′,5,5′-Tetramethylbenzidine CAS 54827-17-7View Details
54827-17-7 -
 3,3,5,5-Tetramethyl Benzidine (TMB) extrapure AR CAS 54827-17-7View Details
3,3,5,5-Tetramethyl Benzidine (TMB) extrapure AR CAS 54827-17-7View Details
54827-17-7 -
 3,3',5,5'-Tetramethylbenzidine CAS 54827-17-7View Details
3,3',5,5'-Tetramethylbenzidine CAS 54827-17-7View Details
54827-17-7 -
 3,3,5,5-Tetramethyl Benzidine (TMB) pure CAS 54827-17-7View Details
3,3,5,5-Tetramethyl Benzidine (TMB) pure CAS 54827-17-7View Details
54827-17-7 -
 3,3'5,5'-TETRAMETHYLBENZIDINE AR CAS 54827-17-7View Details
3,3'5,5'-TETRAMETHYLBENZIDINE AR CAS 54827-17-7View Details
54827-17-7 -
 3,3,5,5-TetramethylbenzidineView Details
3,3,5,5-TetramethylbenzidineView Details
54827-17-7
