Benzidine
Synonym(s):4,4′-Diaminobiphenyl
- CAS NO.:92-87-5
- Empirical Formula: C12H12N2
- Molecular Weight: 184.24
- MDL number: MFCD00039765
- EINECS: 202-199-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
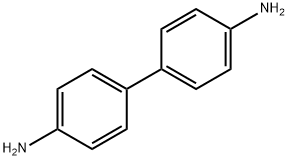
What is Benzidine?
Description
Benzidine is a white, greyish-yellow, or slightly reddish crystalline solid or powder. The major use for benzidine is in the production of dyes, especially azo dyes in the leather, textile, and paper industries and as a synthetic precursor in the preparation and manufacture of dyestuffs. It is also used in the manufacture of rubber, as a reagent, and as a stain in microscopy. It is slightly soluble and slowly changes from a solid to a gas.
Chemical properties
Off-White Solid
Chemical properties
Benzidine is a white, grayish-yellow, or slightly reddish crystalline solid or powder. The major use for benzidine is in the production of dyes, especially azo dyes in the leather, textile, and paper industries, as a synthetic precursor in the preparation and manufacture of dyestuffs. It is also used in the manufacture of dyes and rubber, as a reagent, and as a stain in microscopy. It is slightly soluble and slowly changes from a solid to a gas.
Chemical properties
Benzidine is a white, grayish-yellow crystalline solid or powder. Turns brownish-red on exposure to air and light;
Physical properties
Grayish-yellow to pale reddish powder or crystals. Darkens on exposure to air or light. Odorless.
The Uses of Benzidine
Benzidine was used extensively in the manu facture of dyes. Because of its cancer-causingeffects in humans, its application in dyes hasbeen curtailed. Other uses of this compoundare in chemical analysis: as a reagent for thedetermination of hydrogen peroxide in milkand in the analysis of nicotine. Its hydrochlo ride is used as a reagent to analyze metalsand sulfate.
The Uses of Benzidine
Potentially mutagenic compound.
The Uses of Benzidine
Manufacture of dyestuffs; hardener for rubber; laboratory reagent
Definition
ChEBI: A member of the class of biphenyls that is 1,1'-biphenyl in which the hydrogen at the para-position of each phenyl group has been replaced by an amino group.
Production Methods
Benzidine production is now exclusively for captive consumption and must be carried out in closed systems under stringent workplace controls. Benzidine is used in the synthesisofdyesanddyeintermediates,asahardenerforrubber, and as a laboratory reagent. The ?rst successful synthetic direct dye was Congo Red, a diazo derivative prepared from benzidinebyBoettigerin1884.Nearlyalldirectdyesareazo products. Congo Red is used in humans intravenously for the medical diagnosis of amyloidosis. The basis for its use is an unexplained af?nity for amyloid, which rapidly removes the dye from the blood. It is used medically for the management of profuse capillary hemorrhage such as the one occurring in septicemias and in the terminal phases of leukemia.
Preparation
1-Nitrobenzene restore 1,2-Diphenylhydrazine?turn with acid rearrangement.
Synthesis Reference(s)
The Journal of Organic Chemistry, 41, p. 2661, 1976 DOI: 10.1021/jo00877a041
Synthesis, p. 40, 1976 DOI: 10.1055/s-1976-23952
General Description
A grayish-yellow to grayish-red, crystalline solid. Toxic by ingestion, inhalation, and skin absorption. Combustion produces toxic oxides of nitrogen. Used to make other chemicals and in chemical and biological analysis.
Air & Water Reactions
Darkens on exposure to air and light. Soluble in hot water.
Reactivity Profile
Benzidine forms insoluble salts with sulfuric acid. Can be diazotized, acetylated and alkylated. Is hypergolic with red fuming nitric acid . Neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides.
Hazard
Highly toxic by ingestion, inhalation, and skin absorption. Confirmed carcinogen.
Health Hazard
Poisonous if inhaled, swallowed or absorbed through skin. May cause contact dermatitis, irritation or sensitization. Ingestion may cause nausea and vomiting.
Health Hazard
Exposure to benzidine causes irritation to the eyes. Laboratory animals exposed to benzidine at as low as 0.01% to 0.08% in food showed adverse health effects, such as organ weight decrease in the liver, kidney, and body weight, and an increase in spleen weight, swelling of the liver, and blood in the urine. Exposure may cause an increase in urination, blood in the urine, and urinary tract tumors. Benzidine is considered acutely toxic to humans by ingestion, with an estimated oral lethal dose of between 50 and 500 mg/kg. The symptoms of acute ingestion exposure include cyanosis, headache, mental confusion, nausea, and vertigo. Dermal exposure may cause skin rashes and irritation. Prolonged exposure to benzidine causes bladder injury in humans
Health Hazard
Benzidine is a known carcinogen, causingbladder cancer in humans. Numerous reportsin the literature document its carcinogenicityin animals and humans. Oral or subcutaneousapplication of this compound in experimentalanimals produced tumors in liver, blood,lungs, and skin. The routes of entry intohuman body are primarily the inhalation of its dusts and absorption through skin. Whilehumans and dogs develop bladder cancerfrom benzidine, rodents primarily developliver cancer.Relatively little information is availableon the noncancer health hazard from ben zidine. The acute oral toxicity in animalswas moderate. Ingestion can produce nausea,vomiting, kidney, and liver damage. The oralLD50 values in test animals were in the range150–300 mg/kg.The mechanism of carcinogenicity of ben zidine is thought to involve its metabolictransformations forming reactive intermedi ates binding to DNA. Such DNA adductshave been identified in rodent liver. It testedpositive in most genotoxic tests. Its car cinogenicity may possibly be related to theslow rate of liver detoxification by acetyla tion allowing activation of benzidine or itsmetabolites in urine (Whysner et al. 1996)..
Safety Profile
Confirmed human carcinogen producing bladder tumors. Experimental carcinogenic and tumorigenic data. Poison by ingestion and intraperitoneal routes. Human mutation data reported. Can cause damage to blood, including hemolysis and bone marrow depression. On ingestion causes nausea and vomiting, which may be followed by liver and kidney damage. Any exposure is considered extremely hazardous. When heated to decomposition it emits highly toxic fumes of NOx. See also AROMATIC AMINES.
Potential Exposure
Benzidine is used primarily in the manufacture of azo dyestuffs; there are over 250 of these produced. Other uses, including some which may have been discontinued, are in the rubber industry as a hardener; in the manufacture of plastic films; for detection of occult blood in feces, urine, and body fluids; in the detection of H2O2 in milk; in the production of security paper; and as a laboratory reagent in determining HCN, sulfate, nicotine, and certain sugars. No substitute has been found for its use in dyes. Free benzidine is present in the benzidine-derived azo dyes. According to industry, quality control specifications require that the level not exceed 20 ppm and in practice the level is usually below 10 ppm. Regulations in the USA concerning this chemical define strict procedures to avoid worker contact: mixture containing 0.1% or more must be maintained in isolated or closed systems; employees must observe special personal hygiene rules, and certain procedures must be followed in case of emergencies. Some p-phenylenediamine compounds have been used as rubber components, and DFG warns of danger of skin sensitization. Benzidine and dyes metabolized to benzidine: The following three benzidine-based dyes have been tested and found to cause cancer in rodents after oral exposure for 13 weeks (NCI 1978, IARC 1982): C.I. direct black 38 (CAS 1937-37-7) caused liver cancer in rats and mice, mammary-gland cancer in mice, and colon and urinary-bladder cancer in rats. C.I. direct Blue 6 (CAS 2602-46-2) caused liver cancer in rats. C.I. direct brown 95 (CAS 16071-86-6) caused hepatocellular adenoma in the liver and one malignant liver tumor in rats.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Use gastric lavage if ingested followed bysaline catharsis. Medical observation is recommended for24-48 h after breathing overexposure, as pulmonary edemamay be delayed. As first aid for pulmonary edema, a doctoror authorized paramedic may consider administering a corticosteroid spray.
Carcinogenicity
Benzidine is known to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in humans.
Source
Benzidine can enter the environment by transport, use, and disposal, or by dyes and
pigments containing the compound. A photodegradation product of 3,3′-dichlorobenzidine.
Based on laboratory analysis of 7 coal tar samples, benzidine was ND (EPRI, 1990).
Environmental Fate
Biological. In activated sludge, <0.1% mineralized to carbon dioxide after 5 d (Freitag et al.,
1985). Kincannon and Lin (1985) reported a half-life of 76 d when benzidine in sludge was
applied to a sandy loam soil.
Soil. Benzidine was added to different soils and incubated in the dark at 23 °C under a carbon
dioxide-free atmosphere. After 1 yr, 8.3 to 11.6% of the added benzidine degraded to carbon
dioxide primarily by microbial metabolism and partially by hydrolysis (Graveel et al., 1986).
Tentatively identified biooxidation compounds using GC/MS include hydroxybenzidine, 3-
hydroxybenzidine, 4-amino-4′-nitrobiphenyl, N,N′-dihydroxybenzidine, 3,3′-dihydroxybenzidine
and 4,4′-dinitrobiphenyl (Baird et al., 1977). Under aerobic conditions, the half-life was estimated
to be 2 to 8 d (Lu et al., 1977).
Chemical/Physical. Benzidine is not subject to hydrolysis (Kollig, 1993). Reacts with HCl
forming a salt (C12H12N2?2HCl) that is very soluble in water (61.7 mg/L at 25 °C) (Bowman et al.,
1976).
storage
Benzidine should be kept stored in a cool, well-ventilated area, in closed, sealed containers and out of sunlight and away from heat.
Shipping
UN1885 Benzidine, Hazard Class: 6.1; Labels: 6.1—Poisonous materials. PGII.
Purification Methods
Its solution in *benzene is decolorized by percolating through two 2-cm columns of activated alumina, then concentrated until benzidine crystallises on cooling. Recrystallise alternately from EtOH and *benzene to constant absorption spectrum [Carlin et al. J Am Chem Soc 73 1002 1951]. It has also been crystallised from hot water (charcoal) and from diethyl ether. Dry it under vacuum in an Abderhalden pistol. Store it in the dark in a stoppered container. CARCINOGENIC. [Beilstein 13 IV 364.]
Properties and Applications
white or pink micro crystalline powder. Melting point 125 ℃, boiling point 400 ℃, relative density 1.250 (20 ℃). Soluble in ethanol, rare hydrochloric acid and acetic acid boiling, slightly soluble in ethyl ether, slightly soluble in water, very slightly soluble in cold water. In the air and light color line darker. This product is the dye and organic pigments intermediate.
Toxicity evaluation
Industries release benzidine into the environment in the form of liquid waste and sludges. Benzidine may also be released into the environment due to spillage during transport. In air, benzidine is found bound to suspended particles or as a vapor, which may be brought back to the earth’s surface by rain or gravity.
Incompatibilities
Dust may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. On contact with strong reducing agents, such as hydrides may form flammable gases. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Contact with red fuming nitric acid may cause fire. Oxidizes in air. Neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides.
Waste Disposal
Incineration; oxides of nitrogen are removed from the effluent gas by scrubber, catalytic or thermal device. Package spill residues and sorbent media in 17 hour epoxy-lined drums and move to an EPA-approved disposal site. Treatment may include destruction by potassium permanganate oxidation, hightemperature incineration, or microwave plasma methods. 398 Benzidine Encapsulation by organic polyester resin or silicate fixation. These disposal procedures should be confirmed with responsible environmental engineering and regulatory officials.
Precautions
At high temperatures, benzidine breaks down and releases highly poisonous fumes. During use and handling, workers should wear butyl rubber gloves, goggles, and full body plastic coveralls and ensure that no skin is exposed.
Properties of Benzidine
| Melting point: | 127-128 °C |
| Boiling point: | 402°C |
| Density | 1.25 |
| vapor pressure | Based on the specific vapor density value of 6.36 (Sims et al., 1988), the vapor pressure wascalculated to be 0.83 at 20 °C. |
| refractive index | 1.6266 (estimate) |
| Flash point: | 11 °C |
| storage temp. | 2-8°C |
| solubility | Soluble in ethanol (U.S. EPA, 1985) and ether (1 g/50 mL) (Windholz et al., 1983) |
| pka | 4.66(at 30℃) |
| form | neat |
| color | Grayish-yellow, crystalline powder; white or sltlyreddish crystals, powder |
| Water Solubility | Sparingly soluble. <0.1 g/100 mL at 22 ºC |
| Merck | 13,1077 |
| BRN | 742770 |
| Henry's Law Constant | (x 10-11 atm?m3/mol):
3.88 at 25 °C (estimated, Howard, 1989) |
| Exposure limits | Because it is a carcinogen and readily
absorbed through skin, no TLV has been
assigned. Exposure should be at an absolute
minimum. Recognized Human Carcinogen (ACGIH); Human Carcinogen (MSHA); Carcinogen (OSHA); Human Sufficient Evidence, Animal Sufficient Evidence (IARC). |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 92-87-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzidine(92-87-5) |
| IARC | 1 (Vol. 29, Sup 7, 99, 100F) 2012 |
| EPA Substance Registry System | Benzidine (92-87-5) |
Safety information for Benzidine
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H350:Carcinogenicity H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Benzidine
| InChIKey | HFACYLZERDEVSX-UHFFFAOYSA-N |
Benzidine manufacturer
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran


You may like
-
 Benzidine 98% (GC) CAS 92-87-5View Details
Benzidine 98% (GC) CAS 92-87-5View Details
92-87-5 -
 Benzidine, 99% (forensic grade) CAS 92-87-5View Details
Benzidine, 99% (forensic grade) CAS 92-87-5View Details
92-87-5 -
 Benzidine, GR CAS 92-87-5View Details
Benzidine, GR CAS 92-87-5View Details
92-87-5 -
 Benzidine 95% CAS 92-87-5View Details
Benzidine 95% CAS 92-87-5View Details
92-87-5 -
 Benzidine CAS 92-87-5View Details
Benzidine CAS 92-87-5View Details
92-87-5 -
 Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
67967-07-1 -
 3-Iodophenylacetic acid 1878-69-9 98+View Details
3-Iodophenylacetic acid 1878-69-9 98+View Details
1878-69-9 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
