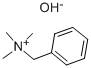
Tetramethylammonium hydroxide
Synonym(s):TMAH solution
- CAS NO.:75-59-2
- Empirical Formula: C4H13NO
- Molecular Weight: 91.15
- MDL number: MFCD00008280
- EINECS: 200-882-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
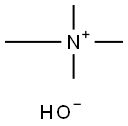
What is Tetramethylammonium hydroxide?
Description
Tetramethylammonium hydroxide is a solid in the hydrated form or a colorless liquid with a strong ammonia-like odor. It is soluble in water, and corrosive to metals and tissue.
Chemical properties
colourless to yellow liquid
The Uses of Tetramethylammonium hydroxide
Tetramethylammonium hydroxide is used to produce tetramethyl-ammonium azide. It is used as an anisotropic etchant of silicon, basic solvent in the development of acidic photoresist in photolithography process, as surfactant in the synthesis of ferrofluid and as a polarographic reagent. It finds application in the production of organic silicon, where it is used for computer silicon wafer surface brightener and cleaning agent. It is also involved in the purification of some metallic elements.
The Uses of Tetramethylammonium hydroxide
The Uses of Tetramethylammonium hydroxide
Tetramethylammonium hydroxide solution (25 wt.% solution in water) may be used as a base for pH adjustment to obtain hexagonal mesoporous aluminophosphate (TAP).
General Description
Tetramethylammonium hydroxide is a quaternary ammonium salt generally used as an anisotropic etchant for silicon due to its high silicon etching rate.
Reactivity Profile
Tetramethylammonium hydroxide acts like a base. Bases are chemically similar to sodium hydroxide (NaOH) or sodium oxide (Na2O). They neutralize acids exothermically to form salts plus water. When soluble in water they give solutions having a pH greater than 7.0. Mixing these materials with water can generate troublesome amounts of heat as the base is dissolved or diluted. Bases react with certain metals (such as aluminum and zinc) to form oxides or hydroxides of the metal and generate gaseous hydrogen. Bases may initiate polymerization reactions in polymerizable organic compounds, especially epoxides). They may generate flammable and/or toxic gases with ammonium salts, nitrides, halogenated organics, various metals, peroxides, and hydroperoxides. Materials of this group often serve as catalysts.
Hazard
Strong irritant to skin and tissue.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Combustible material: may burn but does not ignite readily. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form.
Safety Profile
Poison by subcutaneous route. A powerful caustic. A corrosive irritant to skin, eyes, and mucous membranes. When heated to decomposition it emits toxic fumes of NOx and NH3.
Purification Methods
It is freed from chloride ions by passage through an ion-exchange column (e.g. Amberlite IRA-400, prepared in its OH-form by passing 2M NaOH until the effluent is free from chloride ions, then washed with distilled H2O until neutral). A modification, to obtain carbonate-free hydroxide, uses the method of Davies and Nancollas [Nature 165 237 1950]. [Beilstein 4 IV 145.]
References
1.https://en.wikipedia.org/wiki/Tetramethylammonium_hydroxide#Uses
2.http://sacheminc.com/other-chemicals/tetramethylammonium-hydroxide-tmah/
3.https://www.fs.usda.gov/treesearch/pubs/15506
4.https://www.concordia.ca/content/dam/concordia/services/safety/docs/EHS-DOC-020_TMAHGuidelines.pdf
5.https://www.chemicalbook.com/ChemicalProductProperty_EN_CB2854236.htm
Properties of Tetramethylammonium hydroxide
| Melting point: | °C |
| Boiling point: | 110 °C |
| Density | 0.866 g/mL at 25 °C |
| vapor pressure | 17.5 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 80 °F |
| storage temp. | Store below +30°C. |
| solubility | Methanol (Sparingly), Water (Slightly) |
| form | Solution |
| color | APHA: ≤10 |
| PH | >13 (H2O, 20°C) |
| Odor | strong ammonia-like odor |
| Water Solubility | Soluble in water. |
| Sensitive | Air Sensitive |
| Merck | 14,9224 |
| BRN | 3558708 |
| Stability: | Stable. Flammable. Incompatible with strong oxidizing agents, strong acids. |
| CAS DataBase Reference | 75-59-2(CAS DataBase Reference) |
| EPA Substance Registry System | Tetramethylammonium hydroxide (75-59-2) |
Safety information for Tetramethylammonium hydroxide
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H226:Flammable liquids H314:Skin corrosion/irritation H331:Acute toxicity,inhalation H370:Specific target organ toxicity, single exposure H372:Specific target organ toxicity, repeated exposure H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Tetramethylammonium hydroxide
| InChIKey | WGTYBPLFGIVFAS-UHFFFAOYSA-M |
Tetramethylammonium hydroxide manufacturer
JSK Chemicals
ARRAKIS INDUSTRIES LLP
CureChem India
New Products
(R)-1-Boc-3-hydroxypyrrolidine Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran
You may like
-
 Tetramethylammonium Hydroxide CAS 75-59-2View Details
Tetramethylammonium Hydroxide CAS 75-59-2View Details
75-59-2 -
 Tetramethylammonium hydroxide CAS 75-59-2View Details
Tetramethylammonium hydroxide CAS 75-59-2View Details
75-59-2 -
 Tetramethylammonium hydroxide CAS 75-59-2View Details
Tetramethylammonium hydroxide CAS 75-59-2View Details
75-59-2 -
 Tetramethylammonium hydroxide CAS 75-59-2View Details
Tetramethylammonium hydroxide CAS 75-59-2View Details
75-59-2 -
 Tetramethylammonium Hydroxide 10% aq. solution pure CAS 75-59-2View Details
Tetramethylammonium Hydroxide 10% aq. solution pure CAS 75-59-2View Details
75-59-2 -
 Tetramethylammonium Hydroxide 1M aq. solution ACS CAS 75-59-2View Details
Tetramethylammonium Hydroxide 1M aq. solution ACS CAS 75-59-2View Details
75-59-2 -
 Tetramethylammonium Hydroxide 25% aqueous solution pure CAS 75-59-2View Details
Tetramethylammonium Hydroxide 25% aqueous solution pure CAS 75-59-2View Details
75-59-2 -
 Tetramethylammonium hydroxide solution CASView Details
Tetramethylammonium hydroxide solution CASView Details
