Tetramethylammonium borohydride
Synonym(s):NSC 164901;TMAB
- CAS NO.:16883-45-7
- Empirical Formula: C4H16BN
- Molecular Weight: 88.99
- MDL number: MFCD00011778
- EINECS: 240-917-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
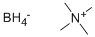
What is Tetramethylammonium borohydride?
The Uses of Tetramethylammonium borohydride
Reactant for:
- Preparation of iridium tetrahydride complexes bearing a tridentate pincer-type bis(phosphino)silyl ligand
- Reduction reactions (reducing agent)
Purification Methods
Recrystallisation of the borohydride from H2O three times yields ca 94% pure compound. Dry in high vacuum at 100o for 3hours. The solubility in H2O is 48% (20o), 61% (40o), and in EtOH 0.5% (25o) and MeCN 0.4% (25o). It decomposes slowly in a vacuum at 150o, but rapidly at 250o. The rate of hydrolysis of Me4N.BH4 (5.8M) in H2O at 40o is constant over a period of 100hours at 0.04% of original wt/hour. The rate decreases to 0.02%/hour in the presence of Me4NOH (5% of the wt of Me4N.BH4). [Banus et al. J Am Chem Soc 74 2346 1952, Beilstein 4 IV 148.]
Properties of Tetramethylammonium borohydride
| Melting point: | 150°C (dec.) |
| Density | 0,813 g/cm3 |
| storage temp. | 2-8°C |
| Water Solubility | almost transparency in Water |
| form | crystal |
| color | white |
| Specific Gravity | 0.813 |
| Sensitive | Moisture Sensitive |
| BRN | 3684968 |
| EPA Substance Registry System | Methanaminium, N,N,N-trimethyl-, tetrahydroborate(1-) (16883-45-7) |
Safety information for Tetramethylammonium borohydride
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H261:Substances And Mixtures Which, In Contact With Water,Emit Flammable Gases H301:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P223:Keep away from any possible contact with water, because of violent reaction and possible flash fire. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P231+P232:Handle under inert gas. Protect from moisture. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Tetramethylammonium borohydride
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

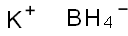
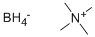
You may like
-
![Tetramethylammonium Borohydride [Reducing Reagent] CAS 16883-45-7](https://img.chemicalbook.in//Content/image/CP5.jpg) Tetramethylammonium Borohydride [Reducing Reagent] CAS 16883-45-7View Details
Tetramethylammonium Borohydride [Reducing Reagent] CAS 16883-45-7View Details
16883-45-7 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1
Statement: All products displayed on this website are only used for non medical purposes such as industrial applications or scientific research, and cannot be used for clinical diagnosis or treatment of humans or animals. They are not medicinal or edible.