Sodium borohydride
Synonym(s):Cobalt-doped sodium borohydride;Sodium borohydride;Sodium tetrahydridoborate;VenPure AF;Vertellus
- CAS NO.:16940-66-2
- Empirical Formula: BH4Na
- Molecular Weight: 37.83
- MDL number: MFCD00003518
- EINECS: 241-004-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:52
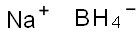
What is Sodium borohydride?
Description
NaBH4 is usually used in hydroxylic solvents such as MeOH, EtOH, and H2O. Sometimes THF is used as a solvent, either alone or as a solvent mixture (ex. THF/MeOH or THF/EtOH). In MeOH and EtOH, NaBH4 decomposes over time to give the respective borates. An excess of NaBH4 can be used to compensate for the decomposition of the reagent over time.
Description
Sodium borohydride (NaBH4) is one of the handiest reducing agents, especially for organic compounds in nonaqueous solvents. It was introduced in the 1940s for wartime applications and was an important enough reagent by 1970 to warrant a lengthy review. It has been used to reduce aldehydes, ketones, Schiff bases, carboxylic acids and esters, acid chlorides, disulfides, nitriles, and inorganic anions. For many of these substrates, only gentle reaction conditions are required.
Chemical properties
Sodium Borohydride is a white, odorless powder or pellet. It is used for bleaching wood pulp, as a blowing agent for plastics, and as a reducing agent for aldehydes and ketones.
Physical properties
White cubic crystals; hygroscopic; density 1.07 g/cm3; decomposes slowly at about 400°C in vacuum or in moist air; soluble in water, decomposing and evolving hydrogen; also soluble in alcohols, liquid ammonia, amines and pyridine.
The Uses of Sodium borohydride
Sodium Borohydride is used as a reagent in the reduction of amino acids and their derivatives. Also used in the catalysis of ammonia borane dehydrogenation.
Reducing agent for aldehydes, ketones and Schiff bases in nonaqueous solvents. Also reduces acids, esters, acid chlorides, disulfides, nitriles, inorganic anions. Further used to generate diborane, as foaming agent, as scavenger for traces of aldehyde, ketones and peroxides in organic chemicals.
Nanocrystalline superlattices in gold colloid solution have been prepared by ligand-induction using AuCl3 reduced with sodium borohydride.1 Nucleophilic addition of hydride ion from sodium borohydride is an inexpensive alternative method for the Baylis-Hillman reaction to form [E]-α-methylcinnamic acids.
The Uses of Sodium borohydride

To a solution of the SM (191 mg, 0.973 mmol) in THF (10 mL) was added NaBH4 (37 mg, 0.978 mmol). The mixture was stirred at RT for 2 h, after which time it was quenched with aq 1N HCl (10 mL) and stirred for 20 min. The resulting mixture was then extracted with DCM (2 x 50 mL). The organic phase was washed with H2O (10 mL), brine (10 mL), dried (Na2SO4), and concentrated in vacuo to provide to product as an off-white solid. [192 mg, 100%]
The Uses of Sodium borohydride
Sodium borohydride is used as a reagent in the reduction of amino acids and their derivatives. Also used as a catalyst in the ammonia borane dehydrogenation. Sodium borohydride is used as a reducing agent in the synthesis of gold nanoparticles. It is also used for wood pulp and as a blowing agent for plastics. It reduces the aldehyde and keto groups to give the corresponding alcohols.
The Uses of Sodium borohydride
Nanocrystalline superlattices in gold colloid solution have been prepared by ligand-induction using AuCl3 reduced with sodium borohydride. Nucleophilic addition of hydride ion from sodium borohydride is an inexpensive alternative method for the Baylis-Hillman reaction to form [E]-a-methylcinnamic acids. A reductant solution composed of NaBH4 and NaOH was used to form mercury cold vapor. NaBH4 may be used to generate hydrogen by catalytic hydrolysis.
What are the applications of Application
Sodium borohydride is a strong reducing agent used in organic and biochemical reactions
Preparation
Sodium borohydride is prepared by reacting sodium hydride with trimethyl borate at about 250°C: 4 NaH + B(OCH3)3 → NaBH3 + 3NaOCH3
Also, sodium borohydride can be made by passing diborane, B2H6, through a solution of sodium methylate, NaOCH3 , in methanol: 2B2H6 + 3NaOCH3→ 3NaBH3 + B(OCH3)3
Alternatively, diborane may be be passed through a solution of sodium tetramethoxyborohydride at low temperatures: 3 NaB(OCH3)3 + 2B2H6 → 3NaBH3 + 4B(OCH3)3.
Production Methods
Sodium bisulfate is a by-product of sodium sulfate manufacture. One process involves reacting sulfuric acid with sodium nitrate at high temperature to form nitric acid and sodium bisulfate: NaNO3 + H2SO3 → NaHSO4 + HNO3(g)
In the above reaction, nitric acid is obtained as vapor. It is purged from the system and collected in water to obtain nitric acid solution of desired concentration. Sodium bisulfate is separated by fractional crystallization.
Definition
ChEBI: Sodium borohydride is an inorganic sodium salt and a metal tetrahydridoborate.
General Description
Sodium borohydride is a white to grayish crystalline powder. Sodium borohydride is decomposed by water to form sodium hydroxide, a corrosive material, and hydrogen, a flammable gas. The heat of this reaction may be sufficient to ignite the hydrogen. The material itself is easily ignited and burns vigorously once ignited. Sodium borohydride is used to make other chemicals, treat waste water, and for many other uses.
Air & Water Reactions
Hydrolysis generates enough heat to ignite adjacent combustible material [Haz. Chem. Data 1966]. Dissolves in water with liberation of heat, may steam and spatter. Solution is basic (alkaline). Reaction of water with the borohydride liberates flammable hydrogen gas. Sodium borohydride burns in air [Lab. Gov. Chemist 1965].
Reactivity Profile
Sodium borohydride is a powerful reducing agent. A chemical base. Absorbs moisture readily forming caustic solution. which attacks aluminum and zinc. A violent polymerization of acetaldehyde results from the reactions of acetaldehyde with alkaline materials such as sodium hydroxide. Calcium oxide or sodium hydroxide react with phosphorus pentaoxide extremely violently when initiated by local heating [Mellor 8 Supp.3:406 (1971]. Using potassium hydroxide to dry impure tetrahydrofuran, which contains peroxides, may be hazardous. Explosions have occurred in the past. Sodium hydroxide behaves in a similar way as potassium hydroxide [NSC Newsletter, Chem. Soc. 1967]. Ignition occurs if a mixture of the hydride and sulfuric acid is not cooled. Contact of glycerol and Sodium borohydride leads to ignition, other glycols and methanol are exothermic but do not ignite.
Hazard
Reacts with water to evolve hydrogen and sodium hydroxide. Flammable, dangerous fire risk. Store out of contact with moisture.
Health Hazard
It is mildly corrosive to skin. Oral intake orintravenous administration of the solid or itssolution produced high toxicity in animals.Ingestion of 160-mg/kg dose was lethal torats (NIOSH 1986).
Fire Hazard
Behavior in Fire: Decomposes and produces highly flammable hydrogen gas.
Flammability and Explosibility
Non flammable
Safety Profile
Poison by ingestion and intraperitoneal routes. A strong alkali. A severe eye, skin, and mucous membrane irritant. Ignites in air above 288’C when exposed to spark. Potentially explosive reaction with aluminum chloride + bis(2-methoxyethyl) ether. Reacts with ruthenium salts to form a solid product which explodes when touched or on contact with water. Reacts to form dangerously explosive hydrogen gas on contact with alkali, water and other protic solvents (e.g., methanol, ethanol, ethylene glycol, phenol), aluminum chloride + bis(2methoxyethy1)ether. Reacts violently with anhydrous acids (e.g., sulfuric, phosphoric, fluorophosphoric) to form diborane. Violent exothermic reaction with dimethyl formamide has caused industrial explosions. Mixtures with sulfuric acid may ignite. Incompatible with palladium, diborane + bis(2-methoxyethyl) ether, polyglycols, dimethylacetamide, oxidizers, metal salts, finely divided metallic precipitates of cobalt, nickel, copper, iron, and possibly other metals. Emits flammable vapors on contact with acid fumes. Materials sensitive to polymerization under alkaline conditions, such as acrylonitrile, may polymerize upon contact with sodium borohydride. Avoid storage in glass containers. When heated to decomposition it emits toxic fumes of NanO. See also HYDRIDES, BORON COMPOUNDS, and SODIUM COMPOUNDS.
Purification Methods
After adding NaBH4 (10g) to freshly distilled diglyme (120mL) in a dry three-necked flask fitted with a stirrer, nitrogen inlet and outlet, the mixture is stirred for 30minutes at 50o until almost all of the solid has dissolved. Stirring is stopped, and, after the solid has settled, the supernatant liquid is forced under N2 pressure through a sintered-glass filter into a dry flask. [The residue is centrifuged to obtain more of the solution which is added to the bulk.] The solution is cooled slowly to 0o and then decanted from the white needles that separated. The crystals are dried by evacuating for 4hours to give anhydrous NaBH4. Alternatively, after the filtration at 50o the solution is heated at 80o for 2hours to give a white precipitate of substantially anhydrous NaBH4 which is collected on a sintered-glass filter under N2, then evacuated at 60o for 2hours [Brown et al. J Am Chem Soc 77 6209 1955]. NaBH4 has also been crystallised from isopropylamine by dissolving it in the solvent at reflux, cooling, filtering and allowing the solution to stand in a filter flask connected to a Dry-ice/acetone trap. After most of the solvent has passed over into the cold trap, crystals are removed with forceps, washed with dry diethyl ether and dried under vacuum. [Kim & Itoh J Phys Chem 91 126 1987.] Somewhat less pure crystals were obtained more rapidly by using Soxhlet extraction with only a small amount of solvent and extracting for about 8hours. The crystals that formed in the flask are filtered off, then washed and dried as before. [Stockmayer et al. J Am Chem Soc 77 1980 1955.] Other solvents used for crystallisation include water and liquid ammonia.
Waste Disposal
It may be destroyed in several ways. Onemethod is as follows (Aldrich 1995). Thesolid or its solution is dissolved or diluted inlarge volume of water. Diluted acetic acid oracetone is then slowly added to this solutionin a well-ventilated area. Hydrogen generatedfrom decomposition of borohydride shouldbe carefully vented out. The pH is adjustedto 1. The solution is then allowed to stand forseveral hours. It is then neutralized to 7, andthe solution is then evaporated to dryness.The residue is then buried in a landfillsite approved for hazardous waste disposal.Sodium borohydride may be destroyed in thelaboratory by alternative methods mentionedfor other hydrides.
Properties of Sodium borohydride
| Melting point: | >300 °C (dec.) (lit.) |
| Boiling point: | 500°C |
| Density | 1.035 g/mL at 25 °C |
| vapor pressure | <1 hPa (25 °C) |
| Flash point: | 158 °F |
| storage temp. | Store at RT. |
| solubility | Methanol (Slightly), Water (Soluble) |
| form | tablets |
| appearance | White solid |
| Specific Gravity | 1.4 |
| color | White |
| PH | 11 (10g/l, H2O, 20℃) |
| explosive limit | 3.02%(V) |
| Water Solubility | 550 g/L (25 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,8592 |
| Stability: | Stability Stable, but reacts readily with water (reaction may be violent). Incompatible with water, oxidizing agents, carbon dioxide, hydrogen halides, acids, palladium, ruthenium and other metal salts, glass. Flammable solid. Air-sensitive. |
| CAS DataBase Reference | 16940-66-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Sodium tetrahydroborate(16940-66-2) |
| EPA Substance Registry System | Sodium borohydride (16940-66-2) |
Safety information for Sodium borohydride
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H260:Substances And Mixtures Which, In Contact With Water,Emit Flammable Gases H301:Acute toxicity,oral H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P280:Wear protective gloves/protective clothing/eye protection/face protection. P231+P232:Handle under inert gas. Protect from moisture. P308+P313:IF exposed or concerned: Get medical advice/attention. P370+P378:In case of fire: Use … for extinction. P402+P404:Store in a dry place. Store in a closed container. |
Computed Descriptors for Sodium borohydride
| InChIKey | YOQDYZUWIQVZSF-UHFFFAOYSA-N |
Sodium borohydride manufacturer
Zama Chemical
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




You may like
-
 Sodium borohydride CAS 16940-66-2View Details
Sodium borohydride CAS 16940-66-2View Details
16940-66-2 -
 Sodium borohydride CAS 16940-66-2View Details
Sodium borohydride CAS 16940-66-2View Details
16940-66-2 -
 Sodium Borohydride CAS 16940-66-2View Details
Sodium Borohydride CAS 16940-66-2View Details
16940-66-2 -
 Sodium Borohydride CAS 16940-66-2View Details
Sodium Borohydride CAS 16940-66-2View Details
16940-66-2 -
 Sodium Borohydride CAS 16940-66-2View Details
Sodium Borohydride CAS 16940-66-2View Details
16940-66-2 -
 Sodium Borohydride CAS 16940-66-2View Details
Sodium Borohydride CAS 16940-66-2View Details
16940-66-2 -
 Sodium Borohydride Powder CASView Details
Sodium Borohydride Powder CASView Details -
 Sodium Borohydride CASView Details
Sodium Borohydride CASView Details
