Tetraterbium heptaoxide
Synonym(s):Terbium peroxide;Tetraterbium heptoxide
- CAS NO.:12037-01-3
- Empirical Formula: O7Tb4-2
- Molecular Weight: 747.7
- MDL number: MFCD00083151
- EINECS: 234-856-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
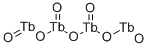
What is Tetraterbium heptaoxide?
Chemical properties
Terbium oxide is a dark brown powder. slightly hygroscopic. Insoluble in water and alkaline solution, soluble in dilute acid. The aqueous suspension is alkaline and can absorb carbon dioxide in the air to form basic carbonate.
Terbium oxide is a functional material used to make metal terbium, magneto-optical glass, phosphors, magneto-optical storage materials, chemical additives, etc.
The Uses of Tetraterbium heptaoxide
Terbium Oxide, also called Terbia, has important role as an activator for green phosphors used in color TV tubes. Meanwhile Terbium Oxide is also used in special lasers and as a dopant in solid-state devices. It is also frequently used as a dopant for crystalline solid-state devices and fuel cell materials. Terbium Oxide is one of the chief commercial Terbium compounds. Produced by heating the metal Oxalate,Terbium Oxide is then used in the preparation of other Terbium compounds.
Preparation
Terbium(III,IV) oxide Synthesis: Tetraterbium nitrate or terbium carbonate or terbium sulfate solution with sodium hydroxide to form terbium hydroxide precipitate, which is filtered and burned to obtain terbium oxide.
4Tb(OH)3+0.5O2→Tb4O7+6H2O
Tb4O7 is most often produced by ignition of the oxalate at or the sulfate in air.
What are the applications of Application
Terbium(III,IV) oxide acts as a precursor for the preparation of other terbium compounds. It is used as a redox catalyst in reactions involving oxygen. At high temperatures, it can liberate oxygen. It finds applications in the manufacture of glass, optical and ceramic. Nanoparticles of terbium oxide are used as analytical reagents for the determination of drugs in food.
Reactions
Terbium Oxide reacts with atomic oxygen to produce TbO2, but more convenient preparations are available. Tb4O7 (s) + 6 HCl (aq) → 2 TbO2 (s) + 2 TbCl3 (aq) + 3 H2O (l) Terbium oxide is heated with ammonium chloride to produce the ammonium salt of the pentachloride: Tb4O7 + 22 NH4Cl → 4 (NH4)2TbCl5 + 7 H2O + 14 NH3
Purification Methods
Dissolve it in acid (e.g. perchloric acid), precipitate it as its oxalate and ignite the oxalate at 650o.
Properties of Tetraterbium heptaoxide
| Melting point: | 2340°C |
| Density | 7.3 g/mL at 25 °C(lit.) |
| storage temp. | Inert atmosphere,Room Temperature |
| form | Powder |
| color | brown to black |
| Specific Gravity | 6.27 |
| Water Solubility | insoluble |
| Merck | 14,9157 |
| CAS DataBase Reference | 12037-01-3(CAS DataBase Reference) |
| EPA Substance Registry System | Terbium oxide (Tb4O7) (12037-01-3) |
Safety information for Tetraterbium heptaoxide
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. P405:Store locked up. |
Computed Descriptors for Tetraterbium heptaoxide
New Products
Tetrabutylammonium iodide (3,3-DIFLUOROCYCLOBUTYL)METHANOL 4,4-DIFLUOROCYCLOHEXANAMINE Cyclobutylamine (S)-3-Fluoro-pyrrolidine hydrochloride 3-Oxocyclobutanecarboxylic acid N-Hydroxy-2-methylpropanimidamide L-tert-Leucine,97% 2-Bromophenylacetonitrile, 97% Aluminum oxide, basic Calcium hydroxide, 95% Diallylamine, 99% 2-Iodobenzoic Acid 3-Methoxybenzonitrile Pentachlorobenzonitrile Chloral Dibenzoyl Peroxide Titanium Dioxide O-Benzylhydroxylamine Hydrochloride 2-Nitrobenzaldehyde 2-Picolylamine (2-Aminomethylpyridine) 2-Venylpyridine Ethyl-2-Chloroacetoacetate 4-Dimethylamine PyridineRelated products of tetrahydrofuran
You may like
-
 Terbium(III,IV) oxide CAS 12037-01-3View Details
Terbium(III,IV) oxide CAS 12037-01-3View Details
12037-01-3 -
 Terbium(III,IV) oxide CAS 12037-01-3View Details
Terbium(III,IV) oxide CAS 12037-01-3View Details
12037-01-3 -
 Terbium(III, IV) oxide CAS 12037-01-3View Details
Terbium(III, IV) oxide CAS 12037-01-3View Details
12037-01-3 -
 Terbium(III, IV) oxide CAS 12037-01-3View Details
Terbium(III, IV) oxide CAS 12037-01-3View Details
12037-01-3 -
 Terbium(III, IV) oxide CAS 12037-01-3View Details
Terbium(III, IV) oxide CAS 12037-01-3View Details
12037-01-3 -
 Terbium(III, IV) oxide CAS 12037-01-3View Details
Terbium(III, IV) oxide CAS 12037-01-3View Details
12037-01-3 -
 Tellurium CAS 12037-01-3View Details
Tellurium CAS 12037-01-3View Details
12037-01-3 -
 Tellurium CAS 12037-01-3View Details
Tellurium CAS 12037-01-3View Details
12037-01-3
