Strontium oxide
Synonym(s):Strontia;Strontium monoxide
- CAS NO.:1314-11-0
- Empirical Formula: SrO
- Molecular Weight: 104.63
- MDL number: MFCD00049558
- EINECS: 215-219-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
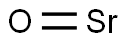
What is Strontium oxide?
Chemical properties
white to grayish white; reacts with water, forming Sr(OH)2, with evolution of heat; enthalpy of fusion 75.00kJ/mol [MER06] [CRC10]
Physical properties
Strontium oxide
or strontia, SrO, is formed when Sr metal reacts with
oxygen:
2Sr+ O2→2SrO
Burning strontium in air results in a mixture of strontium
oxide and strontium nitride. It also forms from the
decomposition of strontium carbonate SrCO3. It is
a strongly basic oxide. It reacts
with moisture forming the hydroxide and with carbon
dioxide in the air to form the carbonate.
The Uses of Strontium oxide
SrO is also used in medicine, pyrotechnics, pigments, greases, soaps, and as a chemical intermediate. It is also produced as high-purity strontium oxide rotatable sputtering targets with the highest possible density and smallest possible average grain sizes for use in semiconductor, photovoltaic, and coating applications by chemical vapor deposition and physical vapor deposition and optical applications.One of the reasons why rare metals are restrained from wide use in production is their high cost.
The Uses of Strontium oxide
manufacture of strontium salts.
The Uses of Strontium oxide
Strontium oxide is used as a flux in the making of glass for television tubes in place of barium oxide [CAS: 1304-28-5] BaO.
Flammability and Explosibility
Not classified
Properties of Strontium oxide
| Melting point: | 2430°C |
| Boiling point: | 3000°C |
| Density | 4.7 g/mL at 25 °C (lit.) |
| Flash point: | 3000°C |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Soluble in potassium hydroxide solution. Slightly soluble in alcohol. Insoluble in acetone and ether. |
| form | Powder |
| Specific Gravity | 4.7 |
| color | white |
| Water Solubility | Soluble in fused potassium hydroxide. Insoluble in ether and acetone. Decomposes in water to Sr(OH){2} with evolution of heat.Slightly soluble in alcohol. Insoluble in acetone and ether. |
| Sensitive | Moisture Sensitive |
| Merck | 14,8846 |
| CAS DataBase Reference | 1314-11-0(CAS DataBase Reference) |
| EPA Substance Registry System | Strontium oxide (SrO) (1314-11-0) |
Safety information for Strontium oxide
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P310:Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Strontium oxide
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Strontium oxide CAS 1314-11-0View Details
Strontium oxide CAS 1314-11-0View Details
1314-11-0 -
 Strontium oxide CAS 1314-11-0View Details
Strontium oxide CAS 1314-11-0View Details
1314-11-0 -
 Strontium oxide CAS 1314-11-0View Details
Strontium oxide CAS 1314-11-0View Details
1314-11-0 -
 Strontium oxide, 99.5% CAS 1314-11-0View Details
Strontium oxide, 99.5% CAS 1314-11-0View Details
1314-11-0 -
 Strontium oxide CAS 1314-11-0View Details
Strontium oxide CAS 1314-11-0View Details
1314-11-0 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1