Tantalum pentoxide
Synonym(s):Tantalum pentoxide
- CAS NO.:1314-61-0
- Empirical Formula: O5Ta2
- Molecular Weight: 441.89
- MDL number: MFCD00011254
- EINECS: 215-238-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-09-04 16:41:41
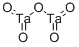
What is Tantalum pentoxide?
Chemical properties
white to white-beige powder
Chemical properties
Tantalum pentoxide, Ta2O5, is a white powder existing in two thermodynamically stable modifications. The orthorombic b-phase changes at 1,360 °C (2,480 °F) into the tetragonal a-modification. The existence of an e-modification has also been reported. Tantalum pentoxide reacts slowly with hot hydrofluoric acid but is insoluble in water and in most solutions of acids and alkalies. For analytical purposes, it can be dissolved by fusion with alkali hydroxides, alkali carbonates, and potassium pyrosulfate.
Physical properties
White orthorhombic crystal or powder; density 8.20 g/cm3; melts at 1,785°C; insoluble in water, ethanol and practically all acids; soluble in hydrofluoric acid; solubilized by fusion with caustic potash or potassium hydrogen sulfate.
Physical properties
Dielectric used in film supercapacitors. Tantalum oxide is a high-refractiveindex, low-absorption material used in making optical coatings in the near-UV (350 nm) to IR (8 μm). Insoluble in most chemicals except HF, HF-HNO3 mixtures, oleum, fused alkali hydroxides (e.g., NaOH, KOH), and molten pyrosulfates.
The Uses of Tantalum pentoxide
Tantalum pentoxide is used in making high refractive index optical glass; as a dielectric film on tantalum for its use as a capacitor component and rectifier; and for preparing tantalum metal, its carbide, and many other tantalum compounds.
The Uses of Tantalum pentoxide
Tantalum oxide is used to make optical glass for lenses and in electronic circuits.
The Uses of Tantalum pentoxide
Tantalum pentoxide (Ta2O5) is used to make special optical glass, for lasers, and in electronic circuits.
Preparation
Tantalum pentoxide is obtained as an intermediate in extracting tantalum from the columbite-tantalite series of minerals. Also, the oxide can be made by heating Ta metal in oxygen or air at elevated temperatures.
General Description
Tantalum pentoxide is dielectric in nature making it useful for making capacitors in the electronics industry.
Hazard
TLV: 5 mg/m3.
Flammability and Explosibility
Non flammable
Safety Profile
Mildly toxic by ingestion. Incompatible with ClF3, BrF3, Li. See also TANTALUM.
Properties of Tantalum pentoxide
| Melting point: | 1872 °C |
| Density | 8.2 g/mL at 25 °C(lit.) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | insoluble in H2O, ethanol, acid solutions; sHF |
| form | lumps |
| color | White to white-beige |
| Specific Gravity | 8.2 |
| Resistivity | 1.0 ∞ 10*12 (ρ/μΩ.cm) |
| Water Solubility | Insoluble in water. |
| Crystal Structure | Trigonal |
| Merck | 14,9056 |
| Exposure limits | OSHA: TWA 5 mg/m3 NIOSH: IDLH 2500 mg/m3; TWA 5 mg/m3; STEL 10 mg/m3 |
| Dielectric constant | 11.6(Ambient) |
| InChI | InChI=1S/5O.2Ta |
| CAS DataBase Reference | 1314-61-0(CAS DataBase Reference) |
| EPA Substance Registry System | Tantalum oxide (Ta2O5) (1314-61-0) |
Safety information for Tantalum pentoxide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H320:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Tantalum pentoxide
| InChIKey | PBCFLUZVCVVTBY-UHFFFAOYSA-N |
| SMILES | [Ta](=O)(=O)O[Ta](=O)=O |
Abamectin manufacturer
JSK Chemicals
Osrics Pharmachem Pvt Ltd (Suryansh Group)
Star Earth Minerals Pvt Ltd
New Products
4-Fluorophenylacetic acid (S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid 5-Aminoimidazole-4-Carbonitrile 4-chloro-3,5-dinitropyridine 2'-Methoxy-biphenyl-2-carboxaldehyde 2-(2-Aminoethyl)isothiourea dihydrobromide, 1-(4-chlorophenyl)propan-1-one 2-Ethyl-4-methyl-1-pentanol DIISOPROPYL MALONATE PENTAFLUOROPHENOL 2-Aminonicotinic acid 6-(4-AMINOPHENYL)-5-METHYL-4,5-DIHYDRO-3(2 H)-PYRDAZINONE β-BUTYROLACTONE 3-OXO-CYCLOBUTANECARBOXYLIC ACID 3-methyl xanthine 1H-Pyrazole-3-carboxylic acid [1,1'-Biphenyl]-4-carboxylic acid (3aR,4R,5R,6aS)-hexahydro-2-oxo-4-[(1E)-3-oxo-4-[3- (trifluoromethyl)phenoxy]-1-buten-1-yl]-2H-cyclopenta[b]furan-5-yl ester 2H-Cyclopenta[b]furan-2,5-diol, hexahydro-4-[(1E,3R)-3-hydroxy-4-[3-(trifluoromethyl)phenoxy]-1- buten-1-yl]-, (3aR,4R,5R,6aS)- 2,5-Dibromopyridine Dimethyl (2-oxo-4-phenylbutyl)phosphonate S-(2-Chloro-3-nitrophenyl) O-ethyl carbonodithioate 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carboxylic acid 2,4-Dichloro-1-[2-nitro-4-(trifluoromethyl)phenoxy]benzeneRelated products of tetrahydrofuran

You may like
-
 Tantalum(V) oxide CAS 1314-61-0View Details
Tantalum(V) oxide CAS 1314-61-0View Details
1314-61-0 -
 Tantalum(V) oxide CAS 1314-61-0View Details
Tantalum(V) oxide CAS 1314-61-0View Details
1314-61-0 -
 Tantalum(V) oxide CAS 1314-61-0View Details
Tantalum(V) oxide CAS 1314-61-0View Details
1314-61-0 -
 Tantalum(V) oxide CAS 1314-61-0View Details
Tantalum(V) oxide CAS 1314-61-0View Details
1314-61-0 -
 Tantalum(V) oxide CAS 1314-61-0View Details
Tantalum(V) oxide CAS 1314-61-0View Details
1314-61-0 -
 Tantalum(V) oxide CAS 1314-61-0View Details
Tantalum(V) oxide CAS 1314-61-0View Details
1314-61-0 -
 Tantalum(V) oxide CAS 1314-61-0View Details
Tantalum(V) oxide CAS 1314-61-0View Details
1314-61-0 -
 Tantalum(V) oxide CAS 1314-61-0View Details
Tantalum(V) oxide CAS 1314-61-0View Details
1314-61-0