Tegaserod
- CAS NO.:145158-71-0
- Empirical Formula: C16H23N5O
- Molecular Weight: 301.39
- MDL number: MFCD06660144
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
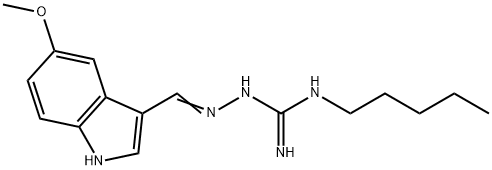
What is Tegaserod?
Absorption
The absolute bioavailability of tegaserod is approximately 10% when administered to fasting subjects. The median time of peak tegaserod plasma concentration (Tmax) is approximately one hour (range 0.7 to 2 hours) .
Nevertheless, when tegaserod was given to individuals thirty minutes before a meal of high-fat and high-calorie content (about 150 calories from protein, 250 calories from carbohydrates, and 500 calories from fat), the AUC was reduced by 40% to 65%, the Cmax was reduced by approximately 20% to 40%, and the median Tmax was 0.7 hours . Additionally, plasma concentrations were similar when tegaserod was administered within thirty minutes before a meal or even two and a half hours after a meal .
Toxicity
Single oral doses of 120 mg (which is 20 times the recommended dose) of tegaserod were administered to three healthy subjects in one study . All three subjects developed diarrhea and headache. Two of these subjects also reported intermittent abdominal pain and one developed orthostatic hypotension . In 28 healthy subjects exposed to 90 to 180 mg per day of tegaserod (which is 7.5 to 15 times the recommended daily dosage) for several days, adverse reactions were diarrhea (100%), headache (57%), abdominal pain (18%), flatulence (18%), nausea (7%), and vomiting (7%) .
Although available data from case reports with tegaserod use in pregnant women have not identified a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes, animal studies involving maternal dietary administration of tegaserod with doses 45 to 71 times the recommended dose demonstrated decreased body weight, delays in developmental landmarks, and decreased survival in rat pups . Caution and careful consideration of risks versus benefits are recommended before administering tegaserod to a pregnant woman.
Despite there being little if any data available regarding the presence of tegaserod in human milk, the effects on the breastfed infant, or the effects on milk production, tegaserod and its metabolites are present in rat milk and the milk to plasma concentration ratio is very high in rats . Subsequently, because of the potential for serious reactions in the breastfed infant, including tumorigenicity, breastfeeding is not recommended during treatment with tegaserod .
The safety and effectiveness of tegaserod in pediatric patients has not yet been established .
Tegaserod is not indicated in patients that are aged 65 years or older .
Tegaserod was not carcinogenic in rats given oral dietary doses up to 180 mg/kg/day (approximately 93 to 111 times the recommended dose based on AUC) for 110 to 124 weeks . In mice, dietary administration of tegaserod for 104 weeks produced mucosal hyperplasia and adenocarcinoma of the small intestine at 600 mg/kg/day (approximately 83 to 110 times the recommended dose based on AUC) . There was no evidence of carcinogenicity at lower doses (3 to 35 times the recommended dose based on AUC) . Tegaserod was not genotoxic in the in vitro Chinese hamster lung fibroblast (CHL/V79) cell chromosomal aberration and forward mutation test, the in vitro rat hepatocyte unscheduled DNA synthesis (UDS) test or the in vivo mouse micronucleus test . The results of the Ames test for mutagenicity were equivocal. Tegaserod at oral (dietary) doses up to 240 mg/kg/day (approximately 57 times the recommended dose based on AUC) in male rats and 150 mg/kg/day (approximately 42 times the recommended dose based on AUC) in female rats was found to have no effect on fertility and reproductive performance .
Inhibition of the hERG (human Ether-a-go-go-Related Gene) channel was evident only in the micromolar concentration range with an IC50 of 13 micromolar (approximately 1300 times the Cmax in humans at the recommended dose) . In in vitro studies, tegaserod had no effects on impulse conduction in isolated guinea pig papillary muscle at up to 100 times the Cmax in humans, Langendorff-perfused isolated rabbit heart (QT interval) at up to 1000 times the Cmax in humans, or human atrial myocytes at multiples up to 10 times the Cmax in humans . The major metabolite, M29, had no effect on QT in the Langendorff-perfused isolated rabbit heart at multiples up to 323 times the Cmax in humans .
In anesthetized and conscious dogs, tegaserod at doses up to 92 to 134 times the recommended dose based on Cmax did not alter heart rate, QRS interval duration, QTc or other ECG parameters . In chronic toxicology studies in rats and dogs, there were no treatment-related changes in cardiac morphology after tegaserod administration at doses up to 660 times the recommended dose based on AUC .
Although tegaserod is expected to bind to 5-HT2B receptors in humans at the recommended dose, there does not appear to be any potential for heart valve injury based on functional evidence of 5-HT2B receptor antagonism .
Studies with isolated coronary and mesenteric blood vessels from non-human primates and humans showed no vasoconstrictor effect at concentrations approximately 100 times the human Cmax . Tegaserod exhibited antagonism of 5-HT-mediated vasoconstriction via 5-HT1B receptors . In rat thoracic aortic rings that were pre-constricted with phenylephrine or norepinephrine, tegaserod produced vasorelaxation, with IC50 values 6 and 64 times the Cmax plasma concentrations in humans, respectively . No effects were observed in the basal tone of aortic rings at concentrations up to 1000 times the human Cmax .
In studies with an anesthetized rat model for measuring macro- and micro-circulation of the colon, intraduodenal dosing with tegaserod (approximately 7 times the recommended dose based on Cmax) produced no clinically relevant effect on blood pressure, heart rate, or vascular conductance .
The Uses of Tegaserod
Treatment of gastrointestinal motility disorders (selective serotonin 5HT4antagonist).
Background
Novartis' brand name Zelnorm (tegaserod) had originally received approval from the US FDA in 2002 for the treatment of irritable bowel syndrome with constipation (IBS-C). It was, however, voluntarily withdrawn from widespread use in the US market in 2007 after concerns arose over the possibility that tegaserod could potentially cause dangerous cardiovascular events in patients. Since then, closer evaluations of the original data suggesting such cardiovascular risk have resulted in the limited reintroduction or 're-approval' of tegaserod for treatment of IBS-C specifically in female patients less than 65 years of age and whom are considered to be at a lower risk of a cardiovascular event than the broader population. Zelnorm (tegaserod) by Sloan Pharma subsequently gained re-approval in April of 2019. Nevertheless, tegaserod remains un-approved in certain regions.
Despite the relative complications involved in its history of regulatory approval, ever since its first introduction in 2002 tegaserod remains the only therapy for IBS-C that possesses the unique mechanism of action of acting on serotonin-4 (5-HT(4)) receptors in smooth muscle cells and in the gastrointestinal wall to facilitate actions like esophageal relaxation, peristaltic gut movement, and natural secretions in the gut, among others.
Indications
Tegaserod is a serotonin-4 (5-HT4) receptor agonist indicated for the treatment of adult women less than 65 years of age with irritable bowel syndrome with constipation (IBS-C) . The safety and effectiveness of tegaserod in men with IBS-C have not been established .
What are the applications of Application
Tegaserod is a serotonin 4 inhibitor
Indications
Tegaserod (Zelnorm) is serotonin-4 (5-HT4) receptor agonists that stimulate GI motility.
Definition
ChEBI: Tegaserod is a member of guanidines, a carboxamidine, a member of hydrazines and a member of indoles. It has a role as a serotonergic agonist and a gastrointestinal drug.
brand name
Zelmac (Novartis).
Pharmacokinetics
In general, it has been determined that tegaserod is an agonist of serotonin type-4 (5-HT(4)) receptors, an antagonist at 5-HT(2B) receptors, but is expected to possess minimal binding to 5-HT(1) receptors, and virtually no affinity for 5-HT(3) or dopamine receptors .
In clinical trials with tegaserod, centrally analyzed ECGs were recorded in 4,605 male and female patients receiving tegaserod 6 mg twice daily or placebo for IBS-C and other related motility disorders . No subject receiving the agent had an absolute QTcF above 480 ms . An increase in QTcF of 30 to 60 ms was observed in 7% of patients receiving tegaserod and 8% receiving placebo . An increase in QTcF of greater than 60 ms was observed in 0.3% and 0.2% of subjects, respectively . The effects of tegaserod on the QTcF interval were ultimately not considered to be clinically meaningful .
Furthermore, it was determined that there is a potential for tegaserod and its main metabolite (the M29 metabolite) to increase platelet aggregation in vitro . In one in vitro study, at concentrations up to 10-times the maximum plasma concentration (Cmax) at the recommended dose, tegaserod significantly increased platelet aggregation in a concentration-dependent manner up to 74% (range 11% to 74%) compared to a control vehicle (with potentiation by various agonists) . In another in vitro study, the M29 metabolite, at concentrations up to 0.6-times the Cmax of M29 also showed a 5% to 16% increase in platelet aggregation compared to the control vehicle . The clinical implications of these in vitro platelet aggregation results remain unclear .
Clinical Use
Tegaserod is being developed as a treatment for constipation- predominant irritable bowel syndrome (IBS). Within the first week, patients treated with tegaserod had significant improvements in abdominal pain and discomfort, constipation, and overall well-being. Efficacy was maintained throughout the treatment period. Tegaserod also demonstrated significant improvements in the three bowel-related assessments (stool frequency, stool consistency, and straining) within the first week, and these improvements were sustained throughout the treatment period. The most common adverse events reported thus far are headache and diarrhea.
Metabolism
Tegaserod is ultimately metabolized by way of hydrolysis and direct glucuronidation . The substance is firstly hydrolyzed in the stomach . It then undergoes oxidization and then conjugation to produce the main circulating tegaserod metabolite in human plasma, the so-called M29 metabolite, or 5-methoxyindole-3-carboxylic acid . Nevertheless, it has been determined that this main circulating metabolite has negligible affinity for 5-HT(4) receptors in vitro . Furthermore, tegaserod can also experience direct N-glucuronidation at each of its three guanidine nitrogens which leads to the generation of three isomeric N-glucuronides - the so-called M43.2, M43.8, and M45.3 metabolites .
Properties of Tegaserod
| Melting point: | 155° |
| Boiling point: | 470.6±48.0 °C(Predicted) |
| Density | 1.18 |
| storage temp. | 2-8°C |
| form | Solid |
| pka | 15.81±0.30(Predicted) |
| color | White to off-white |
| CAS DataBase Reference | 145158-71-0(CAS DataBase Reference) |
Safety information for Tegaserod
Computed Descriptors for Tegaserod
Tegaserod manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 145158-71-0 Tegaserod 98%View Details
145158-71-0 Tegaserod 98%View Details
145158-71-0 -
 145158-71-0 98%View Details
145158-71-0 98%View Details
145158-71-0 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1