Aprepitant
Synonym(s):Aprepitant;Emend;3-[[(2R,3S)-2-[(1R)-1-[3,5-Bis(trifluoromethyl)phenyl]ethoxy]-3-(4-fluorophenyl)morpholino]methyl]-1H-1,2,4-triazol-5(4H)-one;3H-1,2,4-Triazol-3-one, 5-[[(2R,3S)-2-[(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy]-3-(4-fluorophenyl)-4-morpholinyl]methyl]-1,2-dihydro;5-[[(2R,3S)-2-[(1R)-1-[3,5-Bis(trifluoromethyl)phenyl]ethoxy]-3-(4-fluorophenyl)-4-morpholinyl]methyl]-1,2-dihydro-3H-1,2,4-triazol-3-one
- CAS NO.:170729-80-3
- Empirical Formula: C23H21F7N4O3
- Molecular Weight: 534.43
- MDL number: MFCD08277635
- EINECS: 677-636-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 13:58:55

What is Aprepitant?
Absorption
The mean absolute oral bioavailability of aprepitant is approximately 60 to 65%.
Description
Aprepitant is an antiemetic chemical compound that belongs to “substance P” antagonists (SPA) with its effect being blocking the neurokinin 1(Nk1) receptor. It is used for the prevention of acute and delayedchemotherapy-induced nausea and vomiting(CINV) and for prevention ofpostoperative nausea and vomiting. It can also be used for the treatment of cyclic vomiting syndrome and late-stage chemotherapy induced vomiting occurring during cancer treatment. Aprepitant alleviates the case of vomiting in patients through balking the signals released by Nk1 receptors. Nk1 is a G-protein-coupled receptor with its ligand being substance P (SP). The high concentration of SP is required for the vomiting reflex. Aprepitant blocks the process of SP-NK1 signaling in activating the vomiting reflex.
Chemical properties
Off-White to Light Yellow Cyrstalline Solid
Originator
Merck (US)
The Uses of Aprepitant
A novel selective neurokinin-1 (NK-1) receptor antagonist. In vitro studies using human liver microsomes indicate that Aprepitant is metabolised primarily by CYP3A4 with minor metabolism by CYP1A2 and CYP2C19, and no metabolism by CYP2D6, CYP2C9, or CYP2E1. Antiemetic.
The Uses of Aprepitant
anticholinergic
What are the applications of Application
Aprepitant is a selective neurokinin-1 (NK-1) receptor antagonist
Indications
For the prevention of nausea and vomiting associated with highly emetogenic cancer chemotherapy, including high-dose cisplatin (in combination with other antiemetic agents).
Background
Aprepitant, an antiemetic, is a substance P/neurokinin 1 (NK1) receptor antagonist which, in combination with other antiemetic agents, is indicated for the prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of highly emetogenic cancer chemotherapy. Aprepitant is a selective high-affinity antagonist of human substance P/neurokinin 1 (NK1) receptors. Aprepitant has little or no affinity for serotonin (5-HT3), dopamine, and corticosteroid receptors, the targets of existing therapies for chemotherapy-induced nausea and vomiting (CI NV).
Definition
ChEBI: A morpholine-based antiemetic, which is or the prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of highly emetogenic cancer chemotherapy. Aprepitant is a selective high-affinity antagonist of human substance P/ eurokinin 1 (NK1) receptors.
brand name
Emend (Merck).
Pharmacokinetics
Aprepitant, an antiemetic, is a substance P/neurokinin 1 (NK1) receptor antagonist which, in combination with other antiemetic agents, is indicated for the prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of highly emetogenic cancer chemotherapy. Aprepitant is a selective high-affinity antagonist of human substance P/neurokinin 1 (NK1) receptors. Aprepitant has little or no affinity for serotonin (5-HT3), dopamine, and corticosteroid receptors, the targets of existing therapies for chemotherapy-induced nausea and vomiting (CI NV).
Clinical Use
Aprepitant, a substance P (neurokinin-1 [NK-1]) receptor antagonist used for the treatment of chemotherapy-induced nausea and vomiting, was launched in the US and was later approved in the European Union. It is a non-peptide analog having a trisubstituted morpholine with three chiral centers. Two syntheses have been described. In six steps p-fluorophenylacetic acid is converted to 4-benzyl-3-pfluorophenyl- 2-oxomorpholine with a resolution step setting the S-stereochemistry. This intermediate is converted in six steps to aprepitant, with two of the steps utilizing a chiral induction strategy to set the new centers based upon the chiral 2- oxomorpholine intermediate. SAR efforts leading to aprepitant included engineering in potency for NK-1, decreasing affinity for L-type calcium ion channels, most importantly by decreasing the basicity of the core heterocycle. In vitro, it binds with very high affinity (90 pM) to the hNK1 in transfected CHO cells. It is described as an inverse agonist of hNK-1 receptor, with slow dissociation rate under some conditions. In ferrets dosed orally or intravenously prior to emetogen challenge (cisplatin, apomorphine or morphine), retching and vomiting was reduced. Its antiemetic effect is enhanced with the dosing of dexamethasone and it is effective against both the acute and delayed phase of cisplatin-induced emesis. Cisplatin-induced emesis clinical studies showed that aprepitant (125 mg p.o.) in combination with ondansetron (32 mg i.v.) and dexamethasone (20 mg p.o.) therapeutically followed by repeat dosing (days 2–5) of aprepitant (80 mg) dexamethasone (20 mg) provided acute (8 h) and delayed phase (days 2–7) no vomiting rates of 83 and 70%, respectively. L-758298, a prodrug of aprepitant, was not as effective as ondansetron (32 mg i.v.) in reducing acute phase vomiting, but was superior in reducing vomiting in the delayed phase. The terminal half-life range of aprepitant is 9–13 h and the bioavailability is about 65%. It is highly protein bound (95%) and has a Vdss of 70 L. It is a moderate CYP3A4 inhibitor, thus several drugs cleared by CYP3A4 should not be used concurrently. It is also an inducer of CYP2C9 thus potentially modulating the PK of drugs cleared by CYP2C9. Most side effects were mild to moderate, with fatigue, asthenia, diarrhea, and hiccups.
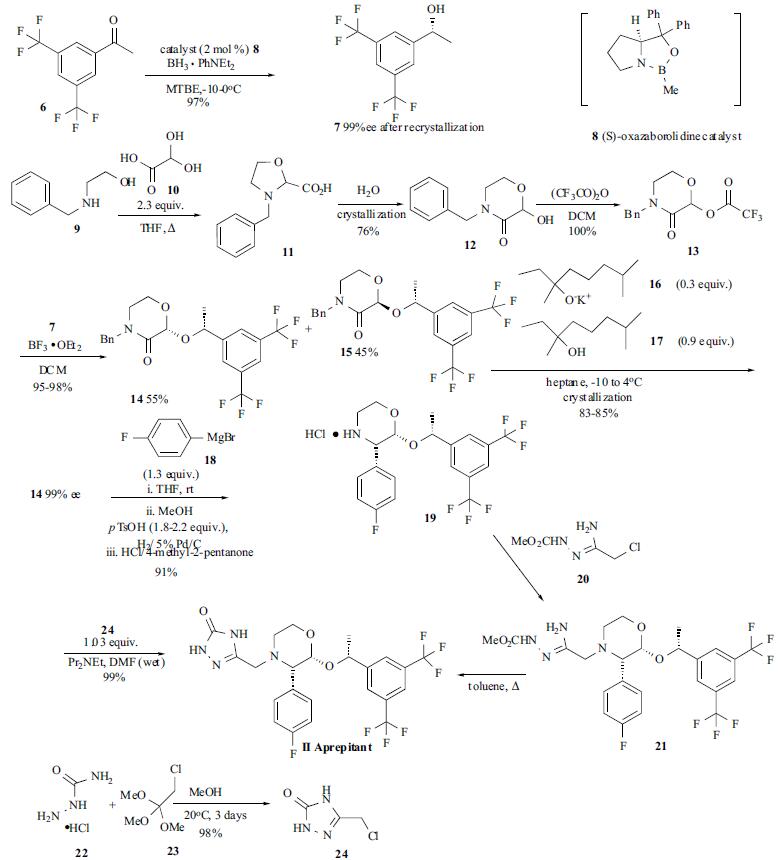
Synthesis
Several
variations to the synthesis of aprepitant (II) have been
published by the Merck group. The latest optimized
synthesis utilizing a novel crystallization-induced
diastereoselective synthesis of aprepitant is highlighted in the
Scheme. The synthetic approach entailed (1) the
synthesis and coupling of the key pieces, N-benzyl lactam
lactol 13 and sec-phenethyl alcohol 7, to provide lactam
acetal 14, (2) stereoselective elaboration to the key
intermediate 14, and (3) conversion to the final compound
via either intramolecular cyclization or intermolecular
coupling with triazolinone chloride 24. The intermediate secphenethyl
alcohol 7 was synthesized in 97% yield and 95%
e.e. (improved to 99% e.e. after recrystallization) via the
enantioselective borane reduction of ketone 6 in the presence
of 2 mol % of (S)-oxazaborolidine catalyst 8. The optimized
conditions involved the slow addition of ketone 6 to a solution containing catalyst 8 and BH3?¤PhNEt2 complex in
MTBE at ¨C10 to 0??C. The synthesis of lactam 12 was done
by reacting N-benzylethanolamine (9) with slight excess of
aqueous glyoxylic acid (10, 2.3 equivalent of 50% aqueous
solution) in refluxing THF. Adjustment of the solvent
composition from predominantly THF to predominantly
water resulted in the crystallization of lactam 12 directly
from 11 in the reaction mixture in 76% yield. Lactam 12
was treated with trifluoroacetic anhydride (1 equiv) to give
trifluoroacetate 13, which was reacted in situ with chiral
alcohol 7 in the presence of BF3?¤OEt2 to give, after workup,
a 55:45 mixture of the acetals 14 and 15 in 95-98% overall
yield. To obtain the desired diastereomer from the 55:45
mixture of 14 and 15, an optimized crystallization sequence
was developed. To a solution of the crude mixture in
heptane, 3,7-dimethyl-3-octanol (17) (0.9 equiv) was added,
cooled to ¨C10 to ¨C5??C and, after seeding the mixture with
pure 14, potassium salt of 3,7-dimethyl-3-octanol (16) (0.3
equiv) was added to initiate the crystallization-induced
epimerization of 15 to 14. After 5 hr, the mixture was
transformed into a 96:4 mixture from which 14 was isolated
in 83-85% yield and £?99% e.e. Under an optimized
condition, the lactam 1 4 was reacted with 4-
fluorophenylmagnesium bromide (18) (1.3 equiv) in THF at
ambient temperature followed by methanol quench and
addition of p-toluenesulfonic acid (1.8-2.2 equiv).
Immediate hydrogenation of this mixture in the presence of
5% Pd/C gave the addition product 19, which was isolated
as hydrochloride salt in 91% yield. Under these conditions,
no cleavage of the benzylic ether group was seen, even after
extended hydrogenation periods. Elaboration to aprepitant
(II) was done by the initial alkylation of 19 in the presence
of a base with amidrazone chloride 20, which was prepared
from chloroacetonitrile, to give the intermediate 21.
Thermolysis of 21 in toluene provided aprepitant (II) in
85% overall yield. Alternatively, the hydrochloride salt 19
has also been alkylated directly with the triazolinone
chloride 24 to give aprepitant (II).
Drug interactions
Potentially hazardous interactions with other drugs
Antidepressants: avoid with St John’s wort.
Antipsychotics: avoid with pimozide.
Avanafil: possibly increases avanafil concentration.
Cytotoxics: possibly increases bosutinib
concentration - avoid or reduce bosutinib dose;
possibly increases ibrutinib concentration - reduce
ibrutinib dose.
Oestrogens and progestogens: may cause
contraceptive failure.
Ulipristal: possibly reduces contraceptive effect -
avoid.
Metabolism
Aprepitant primarily undergoes CYP3A4-mediated metabolism, as well as minor metabolism mediated by CYP1A2 and CYP2C19. About seven metabolites of aprepitant have been identified in human plasma, which all retain weak pharmacological activity.
Metabolism
Aprepitant undergoes extensive metabolism. Following a
single IV 100mg dose of [14C]fosaprepitant, a prodrug for
aprepitant, aprepitant accounts for approximately 19% of
the radioactivity in plasma over 72 hours. 12 metabolites
of aprepitant have been identified in human plasma. The
metabolism of aprepitant, primarily by CYP3A4 and
potentially with minor contribution by CYP1A2 and
CYP2C19, occurs largely via oxidation at the morpholine
ring and its side chains and the resultant metabolites were
only weakly active.
Aprepitant is not excreted unchanged in urine.
Metabolites are excreted in urine (57%) and via biliary
excretion in faeces (45%).
Storage
Store at -20°C
References
Curran, Monique P., and D. M. Robinson. "Aprepitant."Drugs69.13(2009):1853-1878.
Sant P. Chawla M.D. † ‡, et al. "Establishing the dose of the oral NK 1, antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting." Cancer 97.9(2003):2290-2300.
Warr, D. G., et al. "Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer after moderately emetogenic chemotherapy." Journal of Clinical Oncology Official Journal of the American Society of Clinical Oncology23.12(2005):2822-30.
https://en.wikipedia.org/wiki/Aprepitant
Properties of Aprepitant
| Melting point: | 244-246°C |
| alpha | D25 +69° (c = 1.00 in methanol) |
| Density | 1.51±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,2-8°C |
| solubility | Soluble in DMSO (>25 mg/ml) |
| form | powder |
| pka | 8.06±0.20(Predicted) |
| color | white to beige |
| optical activity | [α]/D +61 to +71°, c = 1.0 in methanol |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
| CAS DataBase Reference | 170729-80-3(CAS DataBase Reference) |
Safety information for Aprepitant
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation H351:Carcinogenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. P405:Store locked up. |
Computed Descriptors for Aprepitant
Aprepitant manufacturer
Aquigen Bio science Pvt Ltd
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




You may like
-
 170729-80-3 APREPITANT 43% PELLETS 98%View Details
170729-80-3 APREPITANT 43% PELLETS 98%View Details
170729-80-3 -
 170729-80-3 98%View Details
170729-80-3 98%View Details
170729-80-3 -
 Aprepitant 98%View Details
Aprepitant 98%View Details -
 Aprepitant CAS 170729-80-3View Details
Aprepitant CAS 170729-80-3View Details
170729-80-3 -
 Aprepitant 98% CAS 170729-80-3View Details
Aprepitant 98% CAS 170729-80-3View Details
170729-80-3 -
 Aprepitant 99% (HPLC) CAS 170729-80-3View Details
Aprepitant 99% (HPLC) CAS 170729-80-3View Details
170729-80-3 -
 170729-80-3 Fosaprepitant Impurity C 97 %View Details
170729-80-3 Fosaprepitant Impurity C 97 %View Details
170729-80-3 -
 Aprepitant (125Mg) + Aprepitant (80Mg)(aprecap 125/80 Capsule), For HospitalView Details
Aprepitant (125Mg) + Aprepitant (80Mg)(aprecap 125/80 Capsule), For HospitalView Details
170729-80-3
