Amiloride hydrochloride
Synonym(s):N-Amidino-3,5-diamino-6-chloropyrazinecarboxamide hydrochloride dihydrate;Acid-Sensing Ion Channel Modulator I, ASIC Channel Modulator I;Amiloride hydrochloride dihydrate;Amiloride, Hydrochloride - CAS 2016-88-8 - Calbiochem;N-Amidino-3,5-diamino-6-chloropyrazinecarboxamide hydrochloride hydrate
- CAS NO.:2016-88-8
- Empirical Formula: C6H8ClN7O.ClH
- Molecular Weight: 266.09
- MDL number: MFCD03703482
- EINECS: 217-958-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-04 15:16:13
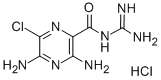
What is Amiloride hydrochloride?
Chemical properties
Pale Yellow Solid
Originator
Midamor,Merck,UK,1971
The Uses of Amiloride hydrochloride
Sodium channel blocker. Diuretic.
The Uses of Amiloride hydrochloride
Na+ channel inhibitor, diuretic
The Uses of Amiloride hydrochloride
A calcium channel andsodium channel protein inhibitor
What are the applications of Application
Amiloride ? HCl is a calcium channel and sodium channel protein inhibitor
Definition
ChEBI: A hydrochloride obtained by combining amiloride with one molar equivalent of hydrochloric acid.
Manufacturing Process
Step A: Preparation of methyl 3-amino-5,6-dichloropyrazinoare: Methyl 3-
aminopyrazinoate (765 g, 5 mols) is suspended in 5 liters of dry benzene.
While stirring under anhydrous conditions sulfuryl chloride (1.99 liters, 3.318
g, 24.58 mols) is added over a period of 30 minutes and stirring is continued
for 1 hour. During this period, the temperature rises to about 50°C and then
begins to drop. The mixture is heated cautiously to reflux (60°C), refluxed for
5 hours and then stirred overnight at room temperature. The excess sulfuryl
chloride is distilled off at atmospheric pressure (distillation is stopped when
vapor temperature reaches 78%). The dark red mixture is chilled to 6°C. The
crystals are filtered off, washed by displacement with two 100 ml portions of
cold (8°C) benzene, then washed with 300 ml petroleum ether and dried in
vacuum at room temperature, yielding 888 g (80%) of methyl 3-amino-5,6-
dichloropyrazinoate in the form of red crystals, MP 228-230°C. The crude
product is dissolved in 56 liters of boiling acetonitrile and passed through a
heated (70-80°C) column of decolorizing charcoal (444 g). The column is
washed with 25 liters of hot acetonitrile, the combined eluate concentrated in
vacuum to about 6 liters and chilled to 5°C. The crystals that form are
filtered, washed three times with cold acetonitrile, and air dried to constant
weight, yielding 724 g (82% recovery, 66% overall) of methyl 3-amino-5,6-
dichloropyrazinoate in the form of yellow crystals, MP 230-234°C. After
additional recrystallizations from acetonitrile the product melts at 233-234°C.
Step B: Preparation of methyl-3,5diamino-6-chloropyrazinoete: In a 2-liter, 3-necked flask fitted with a a mechanical stirrer, thermometer and gas inlet tube
is placed dry dimethyl sulfoxide (1 liter). Methyl 3-amino-5,6-
dichloropyrazinoate (100 g, 0.45 mol) is added and the mixture stirred and
heated at 65°C on a steam bath until solution is effected. A stream of dry
ammonia gas is admitted to the solution with continuous stirring, over a
period of 45 minutes while the temperature is maintained at 65-70°C. The
solution is cooled to about 10°C with continuous stirring and ammonia gas is
admitted for an additional 1 1/4 hours. The yellow reaction mixture is poured,
with stirring, into cold water (2 liters) and the light yellow solid that separates
is removed by filtration, thoroughly washed with water, and dried in a vacuum
desiccator to give 82.5 g (91%) of methyl 3,5-diamino-6-chloropyrazinoate,
MP 210-212°C. Recrystallization from acetonitrile gives material melting at
212-213°C.
Step C: Preparation of the base: A 300 ml one-necked, round-bottomed flask,
equipped with a water-cooled condenser, calcium chloride tube and magnetic
stirrer is charged with anhydrous methanol (150 ml) and sodium metal (5.75
g, 0.25 g atom). When the reaction is complete, the solution is treated with
dry guanidine hydrochloride (26.3 g, 0.275 mol) and stirred for 10 minutes.
The sodium chloride that forms is removed by filtration. The solution is
concentrated in vacuum to a volume of 30 ml and the residue treated with the
product of Step B, heated one minute on a steam bath and kept at 25°C for 1
hour. The product is filtered, washed well with water, dissolved in dilute
hydrochloric acid and the free base precipitated by addition of sodium
hydroxide to give the amiloride product base, a solid which melts at 240.5-
241.5°C.
To produce the hydrochloride, the base is suspended in water (70 ml) and
treated with sufficient 6 N hydrochloric acid to dissolve the free base. The
solution is filtered and treated with concentrated hydrochloric acid (5 ml). The
hydrochloride salt (22 g, 97%) separates and is recrystallized from water (50
ml) containing concentrated hydrochloric acid (3 ml).
brand name
Midamor (Merck).
Therapeutic Function
Diuretic
General Description
Crystalline solid or very light yellow powder.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
A halogenated amine and amide, acidic salt. In aqueous solution, behaves as a weak acid. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides. Organic amides/imides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx).
Health Hazard
SYMPTOMS: Symptoms of exposure to Amiloride hydrochloride include headache, weakness, fatigue, back, chest, neck, shoulder or extremity pain; nausea, anorexia, vomiting, abdominal pain, hyperkalemia, paresthesias, shock, dizziness, coughing, shortness of breath, depression, nervousness, flaccid paralysis of the extremities, bradycardia, flatulence, skin rash, gas pain, constipation and dyspnea.
Fire Hazard
Flash point data for Amiloride hydrochloride are not available; however, Amiloride hydrochloride is probably combustible.
Biological Activity
Na + channel blocker. Defines the I 2A -amiloride sensitive and I 2B -amiloride insensitive imidazoline binding sites. Also inhibits TRPP3 channels.
Biochem/physiol Actions
Selective T-type calcium channel blocker and blocker of epithelial sodium channel. Selective inhibitor of urokinase plasminogen activator (uPA).
Clinical Use
Oedema
Potassium conservation with thiazide and loop
diuretics
Drug interactions
Potentially hazardous interactions with other drugs
ACE inhibitor and angiotensin-II antagonists:
increased risk of hyperkalaemia and hypotension.
Antibacterials: avoid concomitant use with
lymecycline.
Antidepressants: increased risk of postural
hypotension with tricyclics; enhanced hypotensive
effect with MAOIs.
Antihypertensives: enhanced hypotensive effect.
Ciclosporin: increased risk of hyperkalaemia and
nephrotoxicity.
Cytotoxics: increased risk of nephrotoxicity and
ototoxicity with platinum compounds.
Lithium excretion reduced
NSAIDS: increased risk of hyperkalaemia; increased
risk of nephrotoxicity; antagonism of diuretic effect.
Potassium salts: increased risk of hyperkalaemia.
Tacrolimus: increased risk of hyperkalaemia.
Metabolism
Amiloride is excreted unchanged in the urine. In two studies in which single doses of 14C-Amiloride were used, approximately 50% was recovered in urine and 40% in the faeces within 72 hours.
Storage
Room temperature
Properties of Amiloride hydrochloride
| Melting point: | 293-294°C |
| storage temp. | Store at RT |
| solubility | H2O: 50 mg/mL, clear, yellow-green |
| form | powder |
| color | yellow |
| Water Solubility | <0.1 g/100 mL at 19.5 ºC |
| CAS DataBase Reference | 2016-88-8(CAS DataBase Reference) |
| EPA Substance Registry System | Amiloride hydrochloride (2016-88-8) |
Safety information for Amiloride hydrochloride
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Amiloride hydrochloride
Amiloride hydrochloride manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran



You may like
-
 2016-88-8 Amiloride Hydrochloride 98%View Details
2016-88-8 Amiloride Hydrochloride 98%View Details
2016-88-8 -
 2016-88-8 98%View Details
2016-88-8 98%View Details
2016-88-8 -
 Amiloride HCl dihydrate 98% CAS 2016-88-8View Details
Amiloride HCl dihydrate 98% CAS 2016-88-8View Details
2016-88-8 -
 Amiloride, Hydrochloride CAS 2016-88-8View Details
Amiloride, Hydrochloride CAS 2016-88-8View Details
2016-88-8 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
