Cisapride
Synonym(s):cis-4-Amino-5-chloro-N-[1-[3-(4-fluorophenoxy)propyl]-3-methoxy-4-piperidinyl]-2-methoxybenxamide monohydrate;Acenalin;Cipril;Cisapride monohydrate
- CAS NO.:81098-60-4
- Empirical Formula: C23H29ClFN3O4
- Molecular Weight: 465.95
- MDL number: MFCD03305346
- EINECS: 279-689-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-02-23 21:28:46
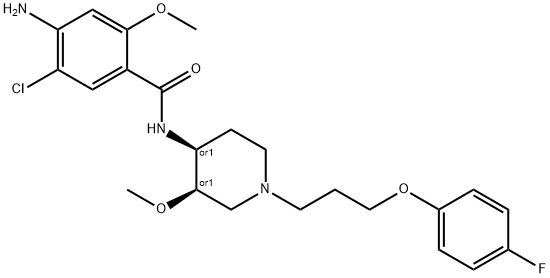
What is Cisapride?
Absorption
Cisapride is rapidly absorbed after oral administration, with an absolute bioavailability of 35-40%.
Description
Cisapride is a gastroprokinetic useful in the treatment of reflux esophagitis, constipation, and a variety of gastro-intestinal motility disorders. Its novel mechanism of action is thought to involve the enhancement of acetylcholine release in the mysenteric plexus of the gut. It is reportedly devoid of CNS and cardiac side-effects.
Chemical properties
White Solid
Originator
Janssen (Belgium)
The Uses of Cisapride
Cisapride Monohydrate is a cardioactive drug that causes prolongation of cardiac repolarization in human; selective serotonin 5-HT4 receptor agonist.
The Uses of Cisapride
A Serotonin 5-HT4 receptro agonist. Used as a Gastroprokinetic
The Uses of Cisapride
Peristaltic stimulant;5-HT antagonist
The Uses of Cisapride
isoflavone used as a cholagogue and cathartic
Indications
For the symptomatic treatment of adult patients with nocturnal heartburn due to gastroesophageal reflux disease.
Background
In many countries (including Canada) cisapride has been either withdrawn or has had its indications limited due to reports about long QT syndrome due to cisapride, which predisposes to arrhythmias. The FDA issued a warning letter regarding this risk to health care professionals and patients.
What are the applications of Application
Cisapride is a SR-4 agonist also known as Enteropride
Definition
ChEBI: The amide resulting from formal condensation of 4-amino-5-chloro-2-methoxybenzoic acid with cis-1-[3-(4-fluorophenoxy)propyl]-3-methoxypiperidin-4-amine. It has been used (as its monohydrate or as its tartrate) for the treatment of gastro-oeso hageal reflux disease and for non-ulcer dyspepsia, but its propensity to cause cardiac arrhythmias resulted in its complete withdrawal from many countries, including the U.K., and restrictions on its use elsewhere.
Indications
Cisapride (Propulsid) is serotonin-4 (5-HT4) receptor agonists that stimulate GI motility. Cisapride appears to act by facilitating the release of acetylcholine from the myenteric plexus. It has no antiadrenergic, antidopaminergic, or cholinergic side effects. Following oral administration, peak plasma levels occur in 1.5 to 2 hours; the drug’s half-life is 10 hours.
Manufacturing Process
A mixture of 1-[3-(4-fluorophenoxy)-propyl]-3-methoxy-4-piperidinone (140
mg), benzylamine (61 mg), Pd 10% on charcoal (100 mg) and a 0.02%
solution of thiophene in THF was reacted under hydrogen gas for 3 hours at
50°C. The catalyst was filtered off and fresh palladium 10% on charcoal (100
mg) was added. Debenzylation of the formed intermediate took place under
hydrogen atmosphere for 18 hours at 50°C. The reaction mixture was filtered
and evaporated under a stream of nitrogen to yield 1-[3-(4-fluorophenoxy)-
propyl]-3-methoxy-4-piperidinamine having a cis/trans ratio of about 93/7.
1-[3-(4-Fluorophenoxy)-propyl]-3-methoxy-4-piperidinamine (50 g) was
dissolved in methyl isobutylketone (250 ml) and a nitric acid solution (65%,
12.8 ml) was carefully added so that the temperature of the solution did not
exceed 45°C. The reaction mixture was stirred at a temperature of 30°C and
seeded. When crystallisation started, the reaction mixture was cooled to 0°C
and stirred for another 2 hours. The product was filtered off, washed with a
small amount of toluene and dried, yielding 1-[3-(4-fluorophenoxy)-propyl]-3-
methoxy-4-piperidinamine nitrate (m.p. 60°C).
1-[3-(4-Fluorophenoxy)-propyl]-3-methoxy-4-piperidinamine nitrate was
dissolved in water (95 ml). The reaction mixture was stirred and toluene (95
ml) was added. A NaOH solution (50%, 10.3 ml) was slowly added and the
temperature of the reaction mixture was raised to 75°C. After 30 min, the
aqueous layer was discarded and the organic layer was evaporated, yielding
1-[3-(4-fluorophenoxy)-propyl]-3-methoxy-4-piperidinamine having a
cis/trans ratio equal to or higher than 98/2.
To a solution of 4-amino-5-chloro-2-methoxybenzoic acid (20.2 g) in methyl
isobutylketone (250 ml) and triethyl amine (15.3 ml) was slowly dropped
ethyl chloroformate (9.6 ml). The reaction mixture was stirred for 30 min at
room temperature. To the formed mixed anhydride was then added 1-[3-(4-
fluorophenoxy)-propyl]-3-methoxy-4-piperidinamine (28.2 g) and the reaction
mixture was stirred for 2 hours at room temperature. Subsequently, the
reaction mixture was washed with water (80 ml) and a NaOH solution (6.5%
w/v, 50 ml). The organic layer was warmed to 65°C and methanol (50 ml)
and water (8.5 ml) were added. The solution was cooled slowly and stirred for
2 days during which crystallisation occurred, yielding benzamide, 4-amino-5-
chloro-N-(1-(3-(4-fluorophenoxy)propyl)-3-methoxy-4-piperidinyl)-2-methoxy-
, monohydrate, cis- (Cisapride) having a cis/trans ratio higher than 99/1.
brand name
Propulsid (Janssen);Calmax;Cisapid;Cismotil;Digenol;Prepulsid.
Therapeutic Function
Gastrointestinal drug
Biological Activity
5-HT 4 receptor agonist and gastrokinetic agent. Stimulates intestinal acetylcholine release, possibly via 5-HT 4 receptor-dependent and -independent mechanisms, leading to increased intestinal motility.
Pharmacokinetics
Cisapride is a parasympathomimetic which acts as a serotonin 5-HT4 agonist; upon activation of the receptor signaling pathway, cisapride promotes the release of acetylcholine neurotransmitters in the enteric nervous system. Cisapride stimulates motility of the upper gastrointestinal tract without stimulating gastric, biliary, or pancreatic secretions. Cisapride increases the tone and amplitude of gastric (especially antral) contractions, relaxes the pyloric sphincter and the duodenal bulb, and increases peristalsis of the duodenum and jejunum resulting in accelerated gastric emptying and intestinal transit. It increases the resting tone of the lower esophageal sphincter. It has little, if any, effect on the motility of the colon or gallbladder. Cisapride does not induce muscarinic or nicotinic receptor stimulation, nor does it inhibit acetylcholinesterase activity.
Clinical Use
Cisapride has been successfully used to treat gastroparesis and mild gastroesophageal reflux disease.
Side Effects
The most frequent side effect has been diarrhea. A few patients had seizure activity that was reversible after medication was discontinued. Cisapride was pulled from the U. S. market after deaths from drug-associated cardiac arrhythmias, including ventricular tachycardia, ventricular fibrillation, torsades de pointes, and QT prolongation.
Veterinary Drugs and Treatments
Proposed uses for cisapride in small animals includes esophageal reflux and treatment of primary gastric stasis disorders. Cisapride has been found to be useful in the treatment of constipation and megacolon in cats.
Metabolic pathway
Cisapride 14C labeled at the carbonyl carbon and 3H labeled at the fluorophenyl moiety or at the piperidine ring is investigated using some animals. N- Dealkylation at the nitrogen of the piperidine ring, resulting in the main urinary metabolite norcisapride, and aromatic hydroxylation of the fluorinated phenyl ring are the major metabolic pathways in dogs and humans. Norcisapride excretion accounts for 14% of the dose in dogs and 41-45% in humans. Minor metabolic pathways are O-dearylation of the 4- fluorophenyl group and oxidation on the piperidine ring (rat, rabbits and dogs: Lavrijsen et al. (1986)15).
Metabolism
Hepatic. Extensively metabolized via cytochrome P450 3A4 enzyme.
storage
Store at RT
Properties of Cisapride
| Melting point: | 109.8 °C |
| Boiling point: | 605.4±55.0 °C(Predicted) |
| Density | 1.29±0.1 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | DMSO: ~30 mg/mL |
| form | solid |
| pka | 13.01±0.40(Predicted) |
| color | white |
| Water Solubility | 9.319mg/L(30 ºC) |
| CAS DataBase Reference | 81098-60-4(CAS DataBase Reference) |
Safety information for Cisapride
Computed Descriptors for Cisapride
Cisapride manufacturer
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran

You may like
-
 17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 -
 131987-69-4 98+View Details
131987-69-4 98+View Details
131987-69-4 -
 2-Hexyn-1-ol 98+View Details
2-Hexyn-1-ol 98+View Details
764-60-3 -
 Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
67967-07-1 -
 764-60-3 2-Hexyn-1-ol 98+View Details
764-60-3 2-Hexyn-1-ol 98+View Details
764-60-3 -
 2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
5968-21-8 -
 3-Iodophenylacetic acid 1878-69-9 98+View Details
3-Iodophenylacetic acid 1878-69-9 98+View Details
1878-69-9 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
