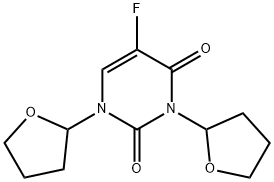
Tegafur
Synonym(s):5-Fluoro-1-(tetrahydro-2-furyl)uracil;Ftorafur;Tegafur
- CAS NO.:17902-23-7
- Empirical Formula: C8H9FN2O3
- Molecular Weight: 200.17
- MDL number: MFCD00012351
- EINECS: 241-846-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
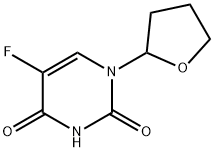
What is Tegafur?
Absorption
Tegafur displays a dose-proportional pharmacokinetic properties. Tegafur is rapidly and well absorbed into the systemic circulation, reaching the peak plasma concentration within 1 to 2 hours of administration .
Toxicity
Oral LD50 value in rat, mouse and dog are 930mg/kg, 775mg/kg, and 34mg/kg, respectively . Continuous exposure to tegafur may cause physical defects in the developing embryo (teratogenesis).
Acute toxicity from the combination use of tegafur was associated with nausea, vomiting, diarrhoea, mucositis, gastrointestinal irritation, bleeding, bone marrow depression, and respiratory failure . Overdose may lead to fatal complications . In case of overdose, appropriate therapeutic and supportive medical interventions should be implemented.
Description
Tegafur is an orally bioavailable prodrug form of 5-fluorouracil (5-FU; ). It is converted to 5-FU in vivo enzymatically or by cytochrome P450 oxidation. Tegafur inhibits proliferation of HT-29, BxPC-3, and Panc02 cells (IC50s = 201, 172, and 179 μM, respectively). It also reduces tumor growth in an HT-27 colon carcinoma mouse xenograft model when administered at a dose of 50 mg/kg and in a 4-1ST gastric carcinoma mouse xenograft model when used in combination with uracil. Formulations containing tegafur, in combination with uracil, have been used in the treatment of cancer.
Chemical properties
White Crystalline Powder
Originator
Futraful,Taiho,Japan,1974
The Uses of Tegafur
Isotope labelled Tegafur (T013900), used as an antineoplastic.
What are the applications of Application
Tegafur is a congener of fluorouracil
Background
Tegafur (INN, BAN, USAN) is a prodrug of Fluorouracil (5-FU), an antineoplastic agent used as the treatment of various cancers such as advanced gastric and colorectal cancers. It is a pyrimidine analogue used in combination therapies as an active chemotherapeutic agent in conjunction with Gimeracil and Oteracil, or along with Fluorouracil as Tegafur-uracil. Tegafur is usually given in combination with other drugs that enhance the bioavailability of the 5-FU by blocking the enzyme responsible for its degradation, or serves to limit the toxicity of 5-FU by ensuring high concentrations of 5-FU at a lower dose of tegafur . When converted and bioactivated to 5-FU, the drug mediates an anticancer activity by inhibiting thymidylate synthase (TS) during the pyrimidine pathway involved in DNA synthesis. 5-FU is listed on the World Health Organization's List of Essential Medicines.
Indications
Indicated for the treatment of cancer usually in combination with other biochemically modulating drugs.
Indicated in adults for the treatment of advanced gastric cancer when given in combination with Cisplatin .
Indicated for the first-line treatment of metastatic colorectal cancer with Uracil and calcium folinate .
Definition
ChEBI: Tegafur is an organohalogen compound and a member of pyrimidines.
Manufacturing Process
One process from US Patent 4,107,162: 27.4 g of 2,4-bis(trimethylsilyl)-5-
fluorouracil and 7.7 g of 2,3-dihydrofuran are dissolved in 70 ml of
acetonitrile, and 30 ml of an acetonitrile solution containing 1.3 g of
anhydrous stannic chloride are added thereinto with cooling and stirring. 50
ml of acetonitrile containing 1.3 ml of water dissolved therein are then
dropwise added over 15 minutes. After return to room temperature, the
reaction is further effected with stirring at 40°C for 5 hours. The reaction
mixture is neutralized by adding 1 N aqueous ammonia with cooling and stirring (conversion 83%). After the nondissolved substances are removed by
filtration, the filtrate is concentrated and dried under reduced pressure. 100
ml of water and 300 ml of dichloromethane are added to the residue to
completely dissolve the residue by stirring. The obtained dichloromethane
layer is separated. The water layer is subjected to extraction twice with
dichloromethane. The thus obtained extracts are combined with the separated
dichloromethane layer and the combined extracts, after drying with anhydrous
magnesium sulfate, are concentrated and dried. The obtained residue is
dissolved in ethanol, and the nondissolved substances are removed by
filtration. The filtrate is subjected to recrystallization to give white crystals,
followed by further recrystallization of the mother liquor. There are totally
obtained 15.6 g of N1-(2'-furanidyl)-5-fluorouracil.Yield: 78% of theory, with
respect to 2,4-bis(trimethylsilyl)-5-fluorouracil.
An alternative process from US Patent 3,635,946: A vigorously stirred reaction
mixture consisting of 32.87 g (0.1 mol) of 5-fluorouracilmercury, 100 ml of
dimethylformamide and 50 ml of toluene is dried by azeotropic distillation of
toluene. It is then cooled to -40°C in a stream of dry nitrogen, and a solution
of 21.3 g (0.2 mol) of 2-chlorofuranidin in 20 ml of dried dimethylformamide
is gradually added to the stirred mixture, the temperature being maintained
between -40°C and -30°C. After completion of the reaction (which is marked
by complete dissolution of the starting 5-fluorouracilmercury) i.e. after about
3 to 4 hours, 60 to 80 ml of the solvent are distilled off in vacuo at a bath
temperature not exceeding 35°C. 50 to 70 ml of dry acetone are then added
and also vacuum distilled. The residue is easily crystallized. It is collected,
washed three times with small quantities of ethanol - 10 ml each - and airdried.
12.2 g of N1-(2'-furanidyl)-5-fluorouracil are obtained in the form of
white crystalline solids; melting point 160°C to 162°C. Additional treatment of
the mother liquor yields 3.0 g more of the product. Yield: 75% of theory,
based on the starting 5-fluorouracilmercury.
After recrystallization from ethanol, 14.3 g of N1-(2'-furanidyl)-5-
fluorouracilare obtained, MP 164°C to 165°C.
Therapeutic Function
Antineoplastic
Biochem/physiol Actions
Tegafur is a pro-drug of 5-fluorouracil, an antimetabolite used as an antineoplastic agent. It has been used as adjuvant chemotherapy for curatively resected colorectal cancer therapy.
Pharmacokinetics
Tegafur is an antineoplastic agent that belongs in the class of pyrimidine analogues. It interferes with the 2'-deoxythymidylate (DTMP) synthesis in the pyrimidine pathway, resulting in inhibition of DNA synthesis . In a phase III trial investigating the clinical efficacy of S-1 (tegafur/gimeracil/oteracil) in patients with advanced or recurrent gastric cancer, treatment resulted in a high response rate and was associated with a longer overall survival and longer progression-free survival rate when used in combination with cisplatin . In a meta analysis, triple combination therapy consisting of tegafur, gimeracil and oteracil showed longer survival times and well tolerance in patients with advanced gastric cancer . Tegafur and its active metabolites are potent myleosuppressive agents .
Safety Profile
Poison by ingestion. Moderately toxic to humans by intravenous route. Moderately toxic experimentally by intraperitoneal, intravenous, and subcutaneous routes. Experimental teratogenic data. Human systemic effects: nausea and vomiting. Experimental reproductive effects. Questionable human carcinogen producing gastrointestinal tumors. Human mutation data reported. Used as an anti-cancer agent. When heated to decomposition it emits very toxic fumes of Fand NOx.
Metabolism
Hepatic CYP2A6 is the predominant enzyme that mediates 5-hydroxylation of tegafur to generate 5'-hydroxytegafur. This metabolite is unstable and undergoes spontaneous degradation to form 5-FU, which is an active antineoplastic agent that exerts a pharmacological action on tumours. 5-FU is rapidly metabolised by the liver enzyme dihydropyrimidine dehydrogenase (DPD) .
Properties of Tegafur
| Melting point: | 171-173 °C(lit.) |
| Density | 1.3222 (estimate) |
| storage temp. | room temp |
| solubility | DMSO: >50mg/mL |
| form | Very Fine Crystalline Powder |
| pka | 7.63±0.10(Predicted) |
| color | white to off-white |
| Merck | 14,9112 |
| CAS DataBase Reference | 17902-23-7(CAS DataBase Reference) |
| EPA Substance Registry System | 2,4(1H,3H)-Pyrimidinedione, 5-fluoro-1-(tetrahydro-2-furanyl)- (17902-23-7) |
Safety information for Tegafur
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Tegafur
| InChIKey | WFWLQNSHRPWKFK-UHFFFAOYSA-N |
Tegafur manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 17902-23-7 Tegafur 98%View Details
17902-23-7 Tegafur 98%View Details
17902-23-7 -
 17902-23-7 98%View Details
17902-23-7 98%View Details
17902-23-7 -
 5-Fluoro-1-(tetrahydro-2-furfuryl)uracil CAS 17902-23-7View Details
5-Fluoro-1-(tetrahydro-2-furfuryl)uracil CAS 17902-23-7View Details
17902-23-7 -
 Ftorafur >99% (HPLC) CAS 17902-23-7View Details
Ftorafur >99% (HPLC) CAS 17902-23-7View Details
17902-23-7 -
 Tegafur CAS 17902-23-7View Details
Tegafur CAS 17902-23-7View Details
17902-23-7 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1