TEBBE REAGENT
Synonym(s):Bis(cyclopentadienyl)-μ-chloro-(dimethylaluminum)-μ-methylenetitanium
- CAS NO.:67719-69-1
- Empirical Formula: C13H4AlClTi
- Molecular Weight: 270.47
- MDL number: MFCD00151575
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-04-03 15:02:28
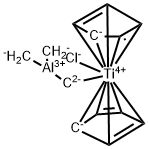
What is TEBBE REAGENT?
Chemical properties
Dark red to purple solution
Chemical properties
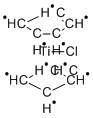
The Tebbe reagent is the organometallic compound with the formula (CH)TiCHClAl(CH). It is used in the methylenation of carbonyl compounds, that is it converts organic compounds containing the RC=O group into the related RC=CH derivative.It is a red solid that is pyrophoric in the air, and thus is typically handled with air-free techniques. It was originally synthesized by Fred Tebbe at DuPont Central Research. Tebbe's reagent contains two tetrahedral metal centers linked by a pair of bridging ligands. The titanium has two cyclopentadienyl ([CH]-, or Cp) rings and aluminium has two methyl groups. The titanium and aluminium atoms are linked together by both a methylene bridge (-CH-) and a chloride atom in a nearly square-planar (Ti–CH–Al–Cl) geometry.The Tebbe reagent was the first reported compound where a methylene bridge connects a transition metal (Ti) and a main group metal (Al).Preparation The Tebbe reagent is synthesized from titanocene dichloride and trimethylaluminium in toluene solution.
The Uses of TEBBE REAGENT
μ-Chloro-μ-methylenebis(cyclopentadienyl)titaniumdimethylaluminum is used in organic synthesis for carbonyl methylenation, A versatile methylenation reagent for the conversion of ketones and aldehydes to olefins, it offers facile reaction with hindered ketones and allows the conversion of esters to vinyl ethers. Tebbe reagent can be used to olefinate aldehydes, to methylenate a chiral polyhydroxyketone with high diasteroselctivity.
The Uses of TEBBE REAGENT
Reagent for the methylenation of carbonyl groups.
The Uses of TEBBE REAGENT
Tebbe reagent can be used:?????
- For the conversion of carbonyl groups of chlorophyll derivatives into the corresponding exo-methylene (or vinylidene) groups.
- In the synthesis of β-C-glycosides from 3-OH glycol esters.
- As a versatile methylenation reagent for the conversion of ketones and aldehydes to olefins. It offers facile reaction with hindered ketones and allows the conversion of esters to vinyl ethers.
- To olefinate aldehydes.
- To methylenate a chiral polyhydroxyketone with high diasteroselctivity.
General Description
Tebbe reagent is a Lewis acid stabilized organometallic compound, which is used to transform carbonyl groups into β-substituted methylenes. It also reacts with a wide range of carbonyl compounds, including esters, amides, and lactones to yield corresponding olefins.
Properties of TEBBE REAGENT
| Density | 0.96 g/mL at 20 °C |
| Flash point: | 34 °F |
| storage temp. | Refrigerator |
| form | Solution |
| color | Dark red to purple |
| Water Solubility | Soluble in toluene, benzene, dichloromethane. Insoluble in water. |
| Sensitive | Air & Moisture Sensitive |
| Merck | 9091 |
| Exposure limits | ACGIH: TWA 20 ppm OSHA: Ceiling 300 ppm; TWA 200 ppm NIOSH: IDLH 500 ppm; TWA 100 ppm(375 mg/m3); STEL 150 ppm(560 mg/m3) |
Safety information for TEBBE REAGENT
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H261:Substances And Mixtures Which, In Contact With Water,Emit Flammable Gases H302:Acute toxicity,oral H304:Aspiration hazard H314:Skin corrosion/irritation H318:Serious eye damage/eye irritation H333:Acute toxicity,inhalation H336:Specific target organ toxicity,single exposure; Narcotic effects H361:Reproductive toxicity H373:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P223:Keep away from any possible contact with water, because of violent reaction and possible flash fire. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P310:Immediately call a POISON CENTER or doctor/physician. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for TEBBE REAGENT
| InChIKey | QEJAQNUJXFLWSP-UHFFFAOYSA-M |
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran


You may like
-
 67719-69-1 Tebbe Reagent 0.5 M in Toluene 99%View Details
67719-69-1 Tebbe Reagent 0.5 M in Toluene 99%View Details
67719-69-1 -
 mu-Chloro-mu-methylenebis (cyclopentadienyl)titaniumdimethylaluminum CAS 67719-69-1View Details
mu-Chloro-mu-methylenebis (cyclopentadienyl)titaniumdimethylaluminum CAS 67719-69-1View Details
67719-69-1 -
 Tebbe reagent solution CAS 67719-69-1View Details
Tebbe reagent solution CAS 67719-69-1View Details
67719-69-1 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4