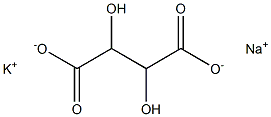
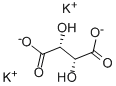
Potassium sodium tartrate
Synonym(s):L -(+)-Tartaric acid potassium Sodium salt solution
- CAS NO.:304-59-6
- Empirical Formula: C4H6O6.K.Na
- Molecular Weight: 212.18
- MDL number: MFCD00065391
- EINECS: 206-156-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
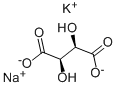
What is Potassium sodium tartrate ?
Description
Potassium sodium tartrate is a double salt first prepared (in about 1675) by an apothecary, Pierre Seignette, of La Rochelle, France. As a result the salt was known as Seignette's salt or Rochelle salt. Rochelle salt is not to be confused with rock salt, which is simply the mineral form of sodium chloride. Potassium sodium tartrate and mono potassium phosphate were the first materials discovered to exhibit piezo electricity.
It is a colorless to blue - white salt crystallizing in the orthorhombic system. Its molecular formula is KNaC4H4O6·4H2O. It is slightly soluble in alcohol but more completely soluble in water. It has a specific gravity of about 1.79, a melting point of approximately 75°C, and has a saline, cooling taste. As a food additive, its E number is E337.
It has been used medicinally as a laxative. It has also been used in the process of silvering mirrors. It is an ingredient of Fehling's solution, formerly used in the determination of reducing sugars in solutions.
Chemical properties
often supplied as a colourless aqueous solution
The Uses of Potassium sodium tartrate
Potassium Sodium Tartrate Solution is a chelator used for research purposes.
The Uses of Potassium sodium tartrate
Laxative.
The Uses of Potassium sodium tartrate
Sodium Potassium Tartrate is a buffer and sequestrant that is the salt of i, (+)–tartaric acid. It has a solubility in water of 1 g in 1 ml. It is also termed rochelle salt and potassium sodium tartrate.
What are the applications of Application
Potassium sodium tartrate solution is a chelator used for research purposes
Definition
ChEBI: The organic sodium and potassium salt of L-tartaric acid (mol ratio 1:1:1).
Preparation
Potassium sodium tartrate, (KNaC4H4O6) may be prepared by adding 0.5 mole sodium carbonate to heated solution containing 1 mole potassium bitartrate(KHC4H5O6). (1M KHC4H4O6 : 0.5M Na2CO3). The solution is filtered while hot. This solution is then dried to precipitate solid potassium sodium tartrate, as small crystallites.
Larger crystals of Rochelle salt have been grown under conditions of reduced gravity and convection on board Skylab .
Flammability and Explosibility
Not classified
Purification Methods
Recrystallise it from distilled water (1.5mL/g) by cooling to 0o. [Beilstein 3 IV 1223.]
Properties of Potassium sodium tartrate
| Melting point: | 70~80℃ |
| Boiling point: | 100 °C |
| Density | 1.24 |
| vapor pressure | 0Pa at 25℃ |
| storage temp. | Room Temperature |
| solubility | Methanol, Water |
| form | Liquid |
| color | Clear Colorless |
| PH | 7.0-8.5 (25℃, 1.5M in H2O) |
| optical activity | [α]20/D +22±1°, c = 1% in H2O |
| Water Solubility | g anhydrous/100 g H2O: 31.9 (0°C), 67.8 (20°C), 102 (30°C) [LAN05]; slightly soluble alcohol [MER06] |
| λmax | λ: 260 nm Amax: 0.03 λ: 280 nm Amax: 0.025 |
| CAS DataBase Reference | 304-59-6(CAS DataBase Reference) |
| EPA Substance Registry System | Butanedioic acid, 2,3-dihydroxy- (2R,3R)-, monopotassium monosodium salt (304-59-6) |
Safety information for Potassium sodium tartrate
Computed Descriptors for Potassium sodium tartrate
Potassium sodium tartrate manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 304-59-6 Sodium Potassium tartrate Solution 98%View Details
304-59-6 Sodium Potassium tartrate Solution 98%View Details
304-59-6 -
 304-59-6 99%View Details
304-59-6 99%View Details
304-59-6 -
 sodium potassium tartarate 98%View Details
sodium potassium tartarate 98%View Details -
 304-59-6 sodium potassium tartarate 98%View Details
304-59-6 sodium potassium tartarate 98%View Details
304-59-6 -
 Fehling's Solution B 99%View Details
Fehling's Solution B 99%View Details
000-01-4 -
 Fehlings solution B CASView Details
Fehlings solution B CASView Details -
 Fehling's Solution B CAS 000-01-4View Details
Fehling's Solution B CAS 000-01-4View Details
000-01-4 -
 Potassium sodium tartrate solution CAS 304-59-6View Details
Potassium sodium tartrate solution CAS 304-59-6View Details
304-59-6
