Trimethylaluminium
Synonym(s):Aluminum trimethanide;Aluminum trimethanide solution;TMA
- CAS NO.:75-24-1
- Empirical Formula: C3H9Al
- Molecular Weight: 72.09
- MDL number: MFCD00008252
- EINECS: 200-853-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
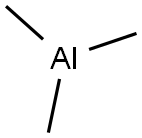
What is Trimethylaluminium?
Chemical properties
The aluminum alkyls are highly flammable and reactive, colorless to yellow liquids at room temperature. The lighter trialkylaluminums ignite spontaneously in air. They are normally supplied and used in a 20% solution with a hydrocarbon solvent, such as hexane, heptane, benzene, toluene. Properties may depend on solvent. Reacts violently with water.
The Uses of Trimethylaluminium
Trimethylaluminium can be used as catalyst for olefin polymerization, pyrophoric fuel, manufacture of straight-chain primary alcohols and olefins, to produce luminous trails in upper atmosphere to track rockets.
The Uses of Trimethylaluminium
Trimethyl aluminum is a highly reactivereducing and alkylating agent. It is used in aZiegler-Natta catalyst for polymerization andhydrogenation.
The Uses of Trimethylaluminium
Trimethylaluminum can be used in the pretreatment of Al2O3/p-type GaSb capacitors.
Definition
A colorless liquid produced by the sodium reduction of dimethyl aluminum chloride. It ignites spontaneously on contact with air and reacts violently with water, acids, halogens, alcohols, and amines. Aluminum alkyls are used in the Ziegler process for the manufacture of high-density polyethene.
Health Hazard
Trimethylaluminum and related alkylaluminum reagents are pyrophoric materials that can react explosively with the moisture in tissues, causing severe burns. The heat of reaction can also ignite the methane gas generated, resulting in thermal burns. Alkylaluminum reagents are corrosive substances, and contact is extremely destructive to the eyes, skin, and mucous membranes. Inhalation of trimethylaluminum and other volatile alkylaluminum compounds may cause severe damage to the respiratory tract and can lead to fatal pulmonary edema.
Health Hazard
As it is pyrophoric and reacts explosivelywith moisture, skin contact can cause a dangerousburn. Contact with eyes can causeblindness. Because of its significant volatility,the risk of inhalation of this compoundis higher than with most other alkyls. Inhalationof its vapors can severely damage therespiratory tract.
TLV-TWA: 2 mg(Al)/m3 (ACGIH).
Flammability and Explosibility
Trimethylaluminum is pyrophoric and burns violently on contact with air or water. Other alkylaluminum reagents show similar behavior, although most are not as volatile as trimethylaluminum. Water or CO2 fire extinguishers must not be used to put out fires involving trialkylaluminum reagents. Instead, dry chemical powders such as bicarbonate, Met-L-X?, or inert smothering agents such as sand or graphite should be used to extinguish fires involving trialkylaluminum compounds.
Potential Exposure
Alkyl aluminum compounds are used as components of olefin polymerization catalysts. They are also used in the synthesis of higher primary alcohols and in pyrophoric fuels, as a catalyst in making ethylene gas; and in plating aluminum.
storage
Safety glasses, impermeable gloves, and a fire-retardant laboratory coat should be worn at all times when working with these compounds. Trialkylaluminum reagents should be handled only under an inert atmosphere.
Shipping
ntial fire or explosion hazard. Shipping: UN3399 Organometallic substance, liquid, water-reactive, flammable, Hazard Class: 4.3; Labels: 4.3 Dangerous Dangerous when wet material, 3-Flammable liquid, technical name Required. UN3051-Spontaneously combustible. Also, this material is dangerous when wet. (Note: this number does not appear in the 49/CFR HazMat tables).
Incompatibilities
The lighter trialkylaluminums ignite spontaneously in air; can self-heat in the air at room temperature without any added energy and may ignite. These compounds are strong reducing agents. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Incompatible with water, oxygen (air), acids, alcohols, phenols, amines, carbon dioxide; sulfur oxides; halogenated compounds, and many other substances
Waste Disposal
Careful incineration
Properties of Trimethylaluminium
| Melting point: | 15 °C |
| Boiling point: | 126 °C |
| Density | 0.81 g/mL at 25 °C |
| vapor pressure | 69.3 mmHg ( 60 °C) |
| Flash point: | 40 °F |
| storage temp. | 0-6°C |
| solubility | Soluble in aromatic, saturated aliphatic and cycloaliphatic hydrocarbons. |
| form | Liquid |
| color | Clear colorless |
| Specific Gravity | 0.688 |
| Odor | Corrosive odor and "taste" may be detectable from trimethylaluminum fires |
| Water Solubility | REACTS |
| Sensitive | Air & Moisture Sensitive |
| Hydrolytic Sensitivity | 9: reacts extremely rapidly with atmospheric moisture - may be pyrophoric - glove box or sealed system required |
| BRN | 3587197 |
| Exposure limits | ACGIH: TWA 50 ppm (Skin) OSHA: TWA 500 ppm(1800 mg/m3) NIOSH: IDLH 1100 ppm; TWA 50 ppm(180 mg/m3) |
| CAS DataBase Reference | 75-24-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Trimethylaluminum(75-24-1) |
| EPA Substance Registry System | Aluminum, trimethyl- (75-24-1) |
Safety information for Trimethylaluminium
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H250:Pyrophoric liquids; Pyrorophoric solids H260:Substances And Mixtures Which, In Contact With Water,Emit Flammable Gases H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P222:Do not allow contact with air. P280:Wear protective gloves/protective clothing/eye protection/face protection. P231+P232:Handle under inert gas. Protect from moisture. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Trimethylaluminium
| InChIKey | JLTRXTDYQLMHGR-UHFFFAOYSA-N |
Trimethylaluminium manufacturer
JSK Chemicals
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 75-24-1 Trimethylaluminum 2.0 M in toluene 99%View Details
75-24-1 Trimethylaluminum 2.0 M in toluene 99%View Details
75-24-1 -
 75-24-1 98%View Details
75-24-1 98%View Details
75-24-1 -
 Trimethyl Aluminium Solution CAS 75-24-1View Details
Trimethyl Aluminium Solution CAS 75-24-1View Details
75-24-1 -
 Trimethylaluminum 2.0 M in toluene CAS 75-24-1View Details
Trimethylaluminum 2.0 M in toluene CAS 75-24-1View Details
75-24-1 -
 Trimethylaluminum solution, 2.0 M in toluene CAS 75-24-1View Details
Trimethylaluminum solution, 2.0 M in toluene CAS 75-24-1View Details
75-24-1 -
 75-24-1View Details
75-24-1View Details
75-24-1 -
 Trimethyl aluminium 2M solution in Toluene CAS 75-24-1View Details
Trimethyl aluminium 2M solution in Toluene CAS 75-24-1View Details
75-24-1 -
 Trimethylaluminum solution CAS 75-24-1View Details
Trimethylaluminum solution CAS 75-24-1View Details
75-24-1