Nysted Reagent
Synonym(s):cyclo-Dibromodi-μ-methylene[μ-(tetrahydrofuran)]trizinc
- CAS NO.:41114-59-4
- Empirical Formula: C6H12Br2OZn3
- Molecular Weight: 456.11
- MDL number: MFCD00134567
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
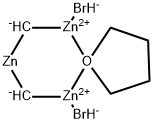
What is Nysted Reagent?
Chemical properties
The Nysted reagent is a reagent used in organic synthesis for the methenylation of a carbonyl group. It was discovered in 1975 by Leonard N. Nysted in Chicago, Illinois. It was originally prepared by reacting dibromomethane and activated zinc in THF.
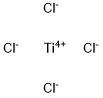
A similar reagent is Tebbe's reagent.In the Nysted olefination, the Nysted reagent reacts with TiCl to methylenate a carbonyl group. The biggest problem with these reagents are that the reactivity has not been well documented. It is believed that the TiCl acts as a mediator in the reaction. Nysted reagent can methylenate different carbonyl groups in the presence of different mediators. For example, in the presence of BF•OEt, the reagent will methylenate aldehydes. On the other hand, in the presence of TiCl, TiCl or TiCl and BF•OEt, the reagent can methylenate ketones. Most commonly, it is used to methylenate ketones because of their general difficulty to methylenate due to crowding around the carbonyl group. The Nysted reagent is able to overcome the additional steric hinderance found in ketones, and more easily methylenate the carbonyl group.
There is little research on Nysted reagent because of the hazards and high reactivity and the difficulty of keeping the reagent stable while it is in use. More specifically, it can form explosive peroxides when exposed to air and is extremely flammable. Also, it reacts violently with water. These make this reagent very dangerous to work with.
The Uses of Nysted Reagent
The cyclo-dibromo-di-m-methylene-(m-tetrahydrofuran)-trizinc is known as the Nysted reagent. This reagent is prepared by treatment of dibromomethane with activated zinc-lead couple or hydrogen chloride-activated zinc dust in THF. This reagent is useful for converting a carbonyl group into a methylene group, and such transformation is known as the Nysted methylenation or Nysted olefination. The study finds that this reagent is superior to the Tebbe reagent.
The Uses of Nysted Reagent
Reagent for the methylenation of ketones and aldehydes.
The Uses of Nysted Reagent
Nysted Reagent is a reagent used in synthetic reactions such as the synthesis of Entcavir.
What are the applications of Application
Nysted Reagent is a useful reagent in the methylenation of ketones
What are the applications of Application
Useful reagent for the methylenation of ketones and α-ketols.
Reactant for:
Synthesis of amphidinolide T3 using a ring-closing metathesis and asymmetric dihydroxylation strategy
Methylenation of carbonyl compounds
Olefination reactions
Reactions
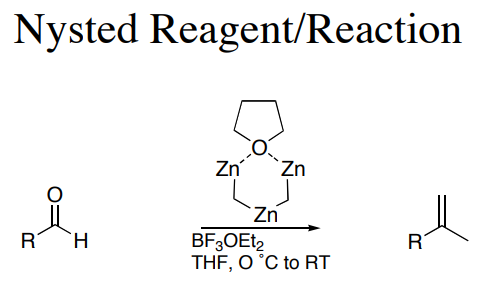
This commerically available reagent is capable of methyenylation alone or in concert with TiCl4. While information on the reactivity of the reagent exists, the mechanistic basis of its function has yet to be elucidated.
Properties of Nysted Reagent
| Density | 1.186 g/mL at 25 °C(lit.) |
| Flash point: | −15 °F |
| CAS DataBase Reference | 41114-59-4 |
Safety information for Nysted Reagent
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H336:Specific target organ toxicity,single exposure; Narcotic effects H351:Carcinogenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Nysted Reagent
| InChIKey | CCTHTLJWXPUNGT-UHFFFAOYSA-L |
Related products of tetrahydrofuran



You may like
-
 Nysted Reagent CAS 41114-59-4View Details
Nysted Reagent CAS 41114-59-4View Details
41114-59-4 -
 37951-47-6 3'-Benzyloxy propiophenone, 98% 99%View Details
37951-47-6 3'-Benzyloxy propiophenone, 98% 99%View Details
37951-47-6 -
 99-30-9 99%View Details
99-30-9 99%View Details
99-30-9 -
 104944-18-5 99%View Details
104944-18-5 99%View Details
104944-18-5 -
 3'-Methoxypropiophenone, 99% 37951-49-8 99%View Details
3'-Methoxypropiophenone, 99% 37951-49-8 99%View Details
37951-49-8 -
 98-56-6 99%View Details
98-56-6 99%View Details
98-56-6 -
 694-48-4 99%View Details
694-48-4 99%View Details
694-48-4 -
 51364-51-3 99%View Details
51364-51-3 99%View Details
51364-51-3