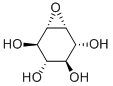
SWAINSONINE
Synonym(s):(1S,2R,8R,8aR)-1,2,8-Octahydroindolizidinetriol;(1S,2S,8R,8aR)-Trihydroxyindolizidine, 8aβ-Indolizidine-1α,2α,8β-triol;Swainsonine, Swainsona canescens - CAS 72741-87-8 - Calbiochem
- CAS NO.:72741-87-8
- Empirical Formula: C8H15NO3
- Molecular Weight: 173.21
- MDL number: MFCD00017554
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-06-30 15:45:59
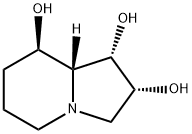
What is SWAINSONINE?
Description
Swainsonine (72741-87-8) is a naturally occurring alkaloidal toxin found in locoweed. Inhibits the biosynthesis of complex glycoproteins by inhibition of Golgi mannosidase II (IC50 = 0.2 mM).1 Inhibits growth and potentiates the cytotoxic effect of taxol in hepatocellular carcinoma in vivo.2 Induces apoptosis in a variety of cell types including cerebral cortical neurons.3 Impairs adult neurogenesis and spatial learning and memory.4? Abrogation of complex glycosylation by swainsonine results in strain-and cell-specific inhibition of prion replication.5 Induces lysosomal storage disease in farm animals.6
Chemical properties
White Crystalline Solid
Occurrence
Swainsonia canescens yields this simple alkaloid.
The Uses of SWAINSONINE
Swainsonine is a plant alkaloid derived from Swainsona canescens (a leguminous plant). It is a reversible, active-site directed inhibitor of a-mannosidase at concentrations of 5-10mM. At acid pH, swainsonine resembles an intermediate in the hydrolysis of mannosidases. Swainsonine completely inhibits mammalian Golgi a-mannosidase II (a-3/6-mannosidase in the glycoprotein processing pathway) and mammalian lysosomal a-mannosidase (acid mannosidase). At higher concentrations, swainsonine also inhibits mammalian cytosolic a-mannosidase. It has been shown to inhibit growth of transformed fibroblasts in soft agar and to enhance the antiproliferative effects of INF on murine lymphoreticular tumor cells in vitro. It also blocks the expression of b1-6 branched complex-type oligosaccharides and shunts the pathway towards hybrid-type oligosaccharides. Swainsonine does not appear to inhibit secretion or expression of glycoproteins at the cell surface. Swainsonine is stable for at least 24 h at 37oC in culture media at physiological pH. Working concentration range is 17-1700 ng/ml (0.1-10mM). Swainsonine (at 1 mg/ml) is not cytotoxic and does not inhibit the growth of a variety of mammalian cell lines. Concentrations required to inhibit Golgi a-mannosidase II in vivo may be somewhat higher, as swainsonine tends to concentrate in the acid environment of cell lysosomes, where it exists as a charged cation and does not permeate through membranes readily. Swainsonine blocks the processing of high-mannose oligosaccharides to complex oligosaccharides. Glycoproteins synthesized in the presence of swainsonine tend to carry mostly high-mannose and hybrid oligosaccharide chains. With short treatments (<24 h) with the inhibitor, cells may retain some complex glycoproteins due to asynchronous cell growth and glycoprotein synthesis.
The Uses of SWAINSONINE
Swainsonine is an indolizidine alkaloid naturally found in certain plants including locoweed that inhibits N-linked glycoside hydrolases, preventing the processing of asparagine-linked glycoproteins. It reversibly inhibits lysosomal α-mannosidase and Golgi α-mannosidase II (IC50 = 0.2 μM). Swainsonine is used to study the role of N-linked glycosylation in cellular processes and has been shown to have antiproliferative and antimetastatic effects of cancer cells in culture and in mice. The inhibition of α-mannosidase activity in lysosomes produces an accumulation of partially-processed oligosaccharides and glycoproteins, giving rise to lysosomal storage disease. Swainsonine toxicity in herbivores results in a condition known as locoism, characterized by hyperactivity, aggression, stiff and clumsy gait, low head carriage, salivation, seizures, and apparent blindness, culminating in increased miscoordination, weakness and death.
The Uses of SWAINSONINE
Swainsonine is a plant alkaloid derived from Swainsona canescens (a leguminous plant). It is a reversible, active-site directed inhibitor of a-mannosidase at concentrations of 5-10mM. At acid pH, swainsonine resembles an intermediate in the hydrolysis of mannosidases.Swainsonine completely inhibits mammalian Golgi a-mannosidase II (a-3/6-mannosidase in the glycoprotein processing pathway) and mammalian lysosomal a-mannosidase (acid mannosidase). At higher concentrations, swainsonine also inhibits mammalian cytosolic a-mannosidase. It has been shown to inhibit growth of transformed fibroblasts in soft agar and to enhance the antiproliferative effects of INF on murine lymphoreticular tumor cells in vitro. It also blocks the expression of b1-6 branched complex-type oligosaccharides and shun
What are the applications of Application
Swainsonine is a potent α-mannosidase inhibitor and immunomodulator
Definition
ChEBI: An indolizidine alkaloid isolated from the plant Swainsona canescens with three hydroxy substituents at positions 1, 2 and 8.
General Description
Swainsonine is produced by endophytes, plant and insect pathogens. It is synthesized from the pipecolic acid and mevalonic acid. Swainsonine is a water-soluble indole alkaloid.
Biological Activity
Inhibitor of α -mannosidase II which inhibits glycoprotein processing. Displays anticancer and immune modulatory properties.
Biochem/physiol Actions
Swainsonine is a potent α-mannosidase inhibitor. It also has antimetastatic, antiproliferative, and immunomodulatory activity . It also inhibits glycoprotein processing.
storage
Store at -20°C
References
1) Tulsiani et al. (1985), Marked differences in the swainsonine inhibition of rat liver lysomal alpha-D-mannosidase, rat liver Golgi mannosidase II, and jack bean alpha-D-mannosidase; Arch. Biochem. Biophys., 236 427 2) You et al. (2012), Swainsonine inhibits growth and potentiates the cytotoxic effect of paclitaxel in hepatocellular carcinoma in vitro and in vivo; Oncol. Rep., 28 2091 3) Lu et al. (2015), Swainsonine-induced apoptosis pathway in cerebral cortical neurons; Res. Vet. Sci., 102 34 4) Wang et al. (2015), Exposure to swainsonine impairs adult neurogenesis and spatial learning and memory; Toxicol. Lett., 232 263 5) Browning et al. (2011), Abrogation of complex glycosylation by swainsonine results in strain- and cell-specific inhibition of prion replication; J. Biol. Chem., 286 40962 6) Dantas et al. (2007), Swainsonine-induced lysosomal storage disease in goats caused by the ingestion of Turbina cordata in Northeastern Brazil; Toxicon, 49 111
Properties of SWAINSONINE
| Melting point: | 148-149°C |
| Boiling point: | 353.3±21.0 °C(Predicted) |
| Density | 1.38±0.1 g/cm3 (20 ºC 760 Torr) |
| storage temp. | -20°C |
| solubility | H2O: soluble1mg/mL |
| form | lyophilized powder |
| pka | 14.01±0.60(Predicted) |
| color | white to faint yellow |
| Water Solubility | Soluble to 50 mM in water |
| BRN | 4175740 |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO, ethanol or distilled water may be stored at -20°C for up to 3 months. |
| CAS DataBase Reference | 72741-87-8 |
Safety information for SWAINSONINE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H312:Acute toxicity,dermal H332:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for SWAINSONINE
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 Swainsonine CAS 72741-87-8View Details
Swainsonine CAS 72741-87-8View Details
72741-87-8 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1