Mitomycin C
Synonym(s):Mitocin C, MMC;Mitomycin;Mitomycin C, Streptomyces caespitosus - CAS 50-07-7 - Calbiochem
- CAS NO.:50-07-7
- Empirical Formula: C15H18N4O5
- Molecular Weight: 334.33
- MDL number: MFCD00078109
- EINECS: 200-008-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
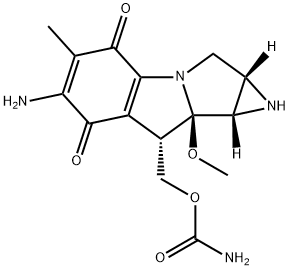
What is Mitomycin C?
Absorption
Erratic.
Toxicity
Oral, mouse: LD50 = 23 mg/kg; Oral, rat: LD50 = 30 mg/kg. Symptoms of overdose include nausea and vomiting.
Description
Mitomycin C is naturally produced by Streptomyces caespitosus, an Actinobacteria found in soil. Mitomycin C has antibiotic and antitumor activities and has been studied extensively since the 1950s. A unique feature of this drug is strong bioreductive alkylation under hypoxic conditions. Oxygen-poor cells internal to solid tumors provide an environment in which this drug is highly activated. As an antitumor agent, it has shown efficacy in a wide variety of cancers, including gastric cancer, pancreatic cancer, breast cancer, non-small-cell lung cancer, cervical cancer, prostate cancer, and bladder cancer. The sideeffect profile is large, which prohibits its widespread use. Mitomycin C is antibacterial to gram-positive, gram-negative, and acid-fast bacilli.
Chemical properties
Blue-violet crystals or crystalline powder.
Chemical properties
Mitomycin is a blue-violet crystalline solid.
Originator
Mitomycin,Medac,W. Germany,1960
The Uses of Mitomycin C
Mitomycin C is the most studied of a family of highly distinctive blue/purple metabolites produced by several Streptomyces species. Mitomyin C exhibits potent antibacterial and antitumour activity and inhibits DNA synthesis by intercalation, blocking nuclear division with the induction of apoptosis in cancer cells.
The Uses of Mitomycin C
An antitumor antibiotic. It is used as antineoplastic.
The Uses of Mitomycin C
Mitomycin C USP (Mutamycin) is used to treat chronic myelogenous leukemia; reticulum cell sarcoma; Hodgkin.s disease; non-Hodgkin.s lymphomas; cancer of stomach, pancreas, lung; epithelial tumors.
Indications
For treatment of malignant neoplasm of lip, oral cavity, pharynx, digestive organs, peritoneum, female breast, and urinary bladder. Also used as an adjunct to ab externo glaucoma surgery. Mitomycin is also indicated as a pyelocalyceal solution for the treatment of adults with low-grade upper tract urothelial cancer (LG-UTUC).
Background
Mitomycin is an antineoplastic antibiotic first isolated by Japanese microbiologists in the 1950s from cultures of Streptomyces caespitosus. It is an alkylating agent that inhibits DNA synthesis (and, at higher concentrations, RNA and protein synthesis) by cross-linking the complementary strands of the DNA double helix. Few other antibiotics have been discovered that work via this alkylating mechanism, making mitomycin relatively unique in the space of microbiota-derived therapies.
Mitomycin's cross-linking activity has resulted in its approval for the treatment of a variety of cancers - the most recent of which is an April 2020 approval for its use in low-grade Upper Tract Urothelial Cancer (LG-UTUC) - as well as adjunctly to ab externo glaucoma surgeries.
What are the applications of Application
Mitomycin C is a DNA crosslinking agent that inhibits DNA synthesis, induces apoptosis in a variety of cells, and activates caspase
What are the applications of Application
Mitomycin C (4% in NaCl) is a DNA crosslinking and damaging agent
Definition
Antibiotic derivedfrom Streptomyces, stated to be effective againsttumors.
Indications
Mitomycin (mitomycin C, Mitocin-C, Mutamycin) is an
antibiotic that is derived from a species of Streptomyces.
It is sometimes classified as an alkylating agent because
it can covalently bind to and cross-link DNA.
Mitomycin is thought to inhibit DNA synthesis through
its ability to alkylate double-strand DNA and bring
about interstrand cross-linking. There is evidence that
enzymatic reduction by a reduced nicotinamide–
adenine dinucleotide phosphate (NADPH) dependent
reductase is necessary to activate the drug.
The drug is rapidly cleared from serum after intravenous
injection but is not distributed to the brain.
Manufacturing Process
The commercial production of mitomycin involves the preparation of
mitomycin-containing broths by culturing a mitomycin-producing organism,
e.g. Streptomyces caespitosus, in suitable media as described at length in the
literature. At the end of the fermentation cycle the whole broth is usually
centrifuged, filtered or otherwise treated to separate the solids (mycelia) from
the supernatant which contains substantially all of the antibiotic activity.
In commercial processes there is usually a period of time intervening between
the end of the fermentation cycle and the time at which the mycelia is
actually removed from the broth; such a period may range from several
minutes to several hours in length and may be due to a number of factors,
e.g., the time necessary to conduct the actual centrifugation or filtration of
large quantities of broth, or the time involved in waiting for equipment to
become available for use. In the commercial preparation of mitomycin, the
mitomycin-containing whole broths decrease rapidly in potency during the
time following the completion of the fermentation cycle and prior to the
removal of the mycelia. It has been observed that a whole broth will lose
substantially all of its mitomycin activity within about 6 hours at room
temperature and within about 24 hours at 10°C. It has, however, been
discovered, as described in US Patent 3,042,582, that in the process for the
recovery of mitomycin C from mitomycin C-containing whole broth, the step of
adding about 0.1 wt % with whole broth of sodium lauryl sulfate to the whole
broth at the completion of the fermentation cycle substantially eliminates such
destruction of mitomycin C by mitase.
brand name
Mutamycin (Bristol-Myers Squibb);Mytozytrex (SuperGen).
Therapeutic Function
Cancer chemotherapy
General Description
Blue-violet crystals. Used as an anti-tumor antibiotic complex.
General Description
administration in the treatment of cancers of the stomachand pancreas when other treatments have failed. Other useshave included breast, NSCLC, cervical, bladder, and headand neck cancers. Mechanisms of resistance include increasedsynthesis of nucleophilic detoxifying compoundssuch as glutathione, decreased expression of activating enzymessuch as DT-diaphorase, and increased efflux by Pgp.The drug is rapidly cleared from the plasma after administrationand widely distributed but does not cross the bloodbrainbarrier. The parent and metabolites are excretedmainly in the feces with an elimination half-life of 50 minutes.Adverse effects include dose-limiting myelosuppression,mild nausea and vomiting,.
General Description
Mitomycin C was isolated from Streptomyces caespitosus in 1958 by Japanese workers and is considered the prototype of the bioreductive alkylating agents. Mitomycin is sometimes included as an alkylating agent but is included here because. It was reasoned that selective activation could be achieved in a reductive environment such as that found in an area of low oxygen content. This is known to occur in tumors where the fast-growing cells often grow beyond the blood supply that would normally provide oxygen. Mitomycin C is capable of being activated and alkylating DNA in an anaerobic environment. The drug contains what would appear to be reactive functionalities, including the quinone and aziridine functionalities, both or which would be thought to be susceptible to nucleophilic attack; however, the reactivity of these functionalities is reduced because of steric and electronic effects in the parent molecule. It was reasoned that selective activation could be achieved in a reductive environment such as that found in an area of low oxygen content. This is known to occur in tumors where the fast-growing cells often grow beyond the blood supply that would normally provide oxygen.A normal cell would undergo apoptosis under these conditions, but because cancer cells often have their apoptotic mechanisms inhibited they continue to survive with little or no oxygen available. Mitomycin C is capable of being activated and alkylating DNA in an anaerobic environment, but there is actually little selectivity for hypoxic cells. Activation can occur enzymatically by both one- and twoelectron processes. Reductive enzymes such as NADPHCYP reductase and DT-diaphorase have been implicated in these processes.Involvement of one-electron processes such as those seen for the anthracylines result in redox cycling and the production of ROS that may result in DNA damage, but the cytotoxicity of mitomycin C is primarily associated with its ability to alkylate DNA.
Air & Water Reactions
Water soluble.
Reactivity Profile
Mitomycin C is sensitive to prolonged exposure to light. Mitomycin C may be sensitive to prolonged exposure to air. Mitomycin C is incompatible with strong oxidizing agents, strong acids and strong bases. Calcium salts may cause decomposition.
Hazard
Possible carcinogen.
Health Hazard
Toxic doses as low as 750 mg/kg have been reported in humans. The major toxic effect is myelosuppression, characterized by marked leukopenia and thrombocytopenia; this may be delayed and cumulative. Interstitial pneumonia and glomerular damage resulting in kidney failure are unusual but well documented complications. Lung conditions -- administration of mitomycin has been recognized as causing pneumonitis, alveolitis and pulmonary fibrosis. Kidney conditions -- administration of Mitomycin Can cause kidney damage. Kidney toxicity was observed in 1-5 percent of patients. Depressed immune conditions.
Fire Hazard
Flash point data for Mitomycin C are not available; however, Mitomycin C is probably combustible.
Biological Activity
Antibiotic and antitumor agent. Covalently binds DNA forming intra- and interstrand crosslinks. Inhibits DNA synthesis.
Pharmacokinetics
Mitomycin is one of the older chemotherapy drugs, which has been around and in use for decades. It is an antibiotic which has been shown to have antitumor activity. Mitomycin selectively inhibits the synthesis of deoxyribonucleic acid (DNA). The guanine and cytosine content correlates with the degree of mitomycin-induced cross-linking. At high concentrations of the drug, cellular RNA and protein synthesis are also suppressed. Mitomycin has been shown in vitro to inhibit B cell, T cell, and macrophage proliferation and impair antigen presentation, as well as the secretion of interferon gamma, TNFa, and IL-2.
Clinical Use
Mitomycin has limited palliative effects in carcinomas of the stomach, pancreas, colon, breast, and cervix.
Side Effects
The major toxicity associated with mitomycin therapy is unpredictably long and cumulative myelosuppression that affects both white blood cells and platelets. A syndrome of microangiopathic hemolytic anemia, thrombocytopenia, and renal failure also has been described. Renal, hepatic, and pulmonary toxicity may occur. The drug is teratogenic and carcinogenic, and it can cause local blistering.
Potential Exposure
This compound is an antitumor antibiotic complex. This drug is usually injected intravenously.
Drug interactions
Potentially hazardous interactions with other drugs
Antipsychotics: avoid with clozapine (increased risk
of agranulocytosis).
Live vaccines: risk of generalised infections - avoid.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit. Keepvictim quiet and maintain normal body temperature.
Environmental Fate
Mitomycin C is naturally produced by S. caespitosus, a microorganism found in soil and decaying vegetation. As a compound potentially released in commercial solid waste or in spill or container residue, mitomycin C is not thought to persist in soil and water. Calculations based on its hydrolysis rate in water at 25 ℃ show a half-life of 12.9 days. It is readily soluble in water, so mobility in groundwater is high. Mitomycin persistence in air is low and bioaccumulation is low.
Metabolism
Primarily hepatic, some in various other tissues.
Metabolism
Mitomycin is administered IV in the treatment of disseminated adenocarcinoma of the stomach or pancreas, and it has been used intravesically in superficial bladder cancer. Biotransformation pathways are saturable, and approximately 10% of an administered dose is eliminated unchanged via the kidneys.
storage
+4°C
Shipping
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required. UN3249 Medicine, solid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Purification Methods
Mitomycin C forms blue-violet crystals from *C6H6/pet ether. It is soluble in Me2CO, MeOH and H2O, moderately soluble in *C6H6, CCl4 and Et2O but insoluble in pet ether. It has UV max at 216, 360 and a weak peak at 560nm in MeOH. [Stevens et al. J Med Chem 8 1 1965, Shirahata & Hirayama J Am Chem Soc 105 7199 1983, Beilstein 25 III/IV 516.]
Toxicity evaluation
Mitomycin C inhibits DNA synthesis and cross-links DNA at the N6 position of adenine and at the O6 and N2 positions of guanine. In addition, single-strand breakage of DNA is caused by reduced mitomycin C (this can be prevented by free radical scavengers). Its action is most prominent during the late G1 and early S phases of the cell cycle. Mitomycin C can inhibit RNA and protein synthesis at high concentrations. Mytomycin C is an aneuploidy-inducing agent. Oxygen and radiation therapy have been shown to enhance the development of toxicity.
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, heat, strong light, calcium salts.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform to EPA regulations governing storage, transportation, treatment, and waste disposal. It is inappropriate and possibly dangerous to the environment to dispose of expired or waste drugs and pharmaceuticals by flushing them down the toilet or discarding them to the trash. Household quantities of expired or waste pharmaceuticals may be mixed with wet cat litter or coffee grounds, double-bagged in plastic, discard in trash. Larger quantities shall carefully take into consideration applicable DEA, EPA, and FDA regulations. If possible return the pharmaceutical to the manufacturer for proper disposal being careful to properly label and securely package the material. Alternatively, the waste pharmaceutical shall be labeled, securely packaged, and transported by a state licensed medical waste contractor to dispose by burial in a licensed hazardous or toxic waste landfill or incinerator.
References
1) Tee and Proud (2000), DNA-damaging agents cause inactivation of translational regulators linked to mTOR signaling; Oncogene, 30 21 2) Park et al. (2000), Mitomycin C induces apoptosis in a caspases-dependent and Fas/CD95- independent manner in human gastric adenocarcinoma cells; Cancer Lett., 158 125 3) Merck Index 14:6215
Properties of Mitomycin C
| Melting point: | 360 °C |
| Boiling point: | 471.14°C (rough estimate) |
| Density | 1.2238 (rough estimate) |
| refractive index | 1.6800 (estimate) |
| storage temp. | 2-8°C |
| solubility | H2O: 4 mL/vial Stock solutions should be filter sterilized and stored at 2-8 °C in the dark., clear to slightly hazy, blue to purple |
| form | powder |
| pka | pKa 2.8(H2O,t =25,I=0.1) (Uncertain) |
| color | blue-gray |
| PH | pH (0.5 g/l, 25℃ : )5.0~7.0 |
| Water Solubility | soluble |
| Merck | 14,6215 |
| BRN | 7231816 |
| Stability: | Stable. Incompatible with strong acids, strong bases, strong oxidizing agents. |
| CAS DataBase Reference | 50-07-7(CAS DataBase Reference) |
| IARC | 2B (Vol. 10, Sup 7) 1987 |
| EPA Substance Registry System | Mitomycin C (50-07-7) |
Safety information for Mitomycin C
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H300:Acute toxicity,oral H351:Carcinogenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Mitomycin C
Related products of tetrahydrofuran







You may like
-
 Mitomycin C CAS 50-07-7View Details
Mitomycin C CAS 50-07-7View Details
50-07-7 -
 Ametycin CAS 50-07-7View Details
Ametycin CAS 50-07-7View Details
50-07-7 -
 Mitomycin C from Streptomyces caespitosus CAS 50-07-7View Details
Mitomycin C from Streptomyces caespitosus CAS 50-07-7View Details
50-07-7 -
 Mitomycin C from Streptomyces caespitosus CAS 50-07-7View Details
Mitomycin C from Streptomyces caespitosus CAS 50-07-7View Details
50-07-7 -
 Mitomycin C from Streptomyces caespitosus CAS 50-07-7View Details
Mitomycin C from Streptomyces caespitosus CAS 50-07-7View Details
50-07-7 -
 Mitomycin C from Streptomyces caespitosus CAS 50-07-7View Details
Mitomycin C from Streptomyces caespitosus CAS 50-07-7View Details
50-07-7 -
 Mitomycin C 98%View Details
Mitomycin C 98%View Details
50-07-7 -
 Pyrrolidine 99.00%View Details
Pyrrolidine 99.00%View Details
123-75-1