Betaine
Synonym(s):(Carboxymethyl)trimethylammonium inner salt;Betaine;Oxyneurine;TMG;Trimethylglycine
- CAS NO.:107-43-7
- Empirical Formula: C5H11NO2
- Molecular Weight: 117.15
- MDL number: MFCD00012123
- EINECS: 203-490-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
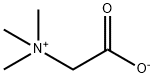
What is Betaine?
Description
"Betaine" is the name of a class of zwitterionic compounds, but it is also commonly used to refer to glycine betaine, or trimethylglycine, pictured. Glycine betaine exists widely in nature; it was first isolated from the sugar beet Beta vulgaris, from which it takes its name. Betaine has many uses, including cosmetics, aircraft deicing, crop protection, fermentation, and animal and human nutrition.
Chemical properties
White cryst. powder
Chemical properties
White crystalline powder; bland aroma.
The Uses of Betaine
Betaine is an active ingredient in toothpaste to control the symptoms of dryness of the mouth. It is used to treat homocystinuria, which is a defect in the major pathway of methionine biosynthesis. It is also used for boosting the immune system and for improving athletic performance. It is helpful to prevent noncancerous tumors in the colon (colorectal adenomas).
The Uses of Betaine
Betaine has been used to study the effects of antioxidants on regrowth from cryopreservation.
The Uses of Betaine
betaine is a surfactant, humectant, and excellent skin conditioner. It is also used to build product viscosity and as a foam booster. It is found mostly in skin cleansers, shampoos, and bath products.
Background
Betaine is a methyl group donor that functions in the normal metabolic cycle of methionine. It is a naturally occurring choline derivative commonly ingested through diet, with a role in regulating cellular hydration and maintaining cell function. Homocystinuria is an inherited disorder that leads to the accumulation of homocysteine in plasma and urine. Currently, no treatments are available to correct the genetic causes of homocystinuria. However, in order to normalize homocysteine levels, patients can be treated with vitamin B6 (pyridoxine), vitamin B12 (cobalamin), folate and specific diets. Betaine reduces plasma homocysteine levels in patients with homocystinuria. Although it is present in many food products, the levels found there are insufficient to treat this condition. The FDA and EMA have approved the product Cystadane (betaine anhydrous, oral solution) for the treatment of homocystinuria, and the EMA has approved the use of Amversio (betaine anhydrous, oral powder).
Indications
Betaine is indicated for the treatment of homocystinuria in pediatric and adult patients to decrease elevated homocysteine blood levels. Included within the category of homocystinuria are deficiencies or defects in cystathionine beta-synthase (CBS), 5,10-methylenetetrahydrofolate reductase (MTHFR), and cobalamin cofactor metabolism (cbl).
Definition
ChEBI: The amino acid betaine derived from glycine.
General Description
Betaine also called trimethylglycine or N,N,N triethylammonium acetate, is an analog of glycine with three methyl groups. It is highly compatible with polymerase chain reaction (PCR) buffer mixture. Betaine is a PCR enhancing reagent that is widely used for improving the yield and specificity of PCR products, especially during the PCR amplification of targets rich in GC content or those that form secondary structures resulting in poor yield. Betaine facilitates DNA strand separation and manages the DNA melting temperature (Tm) difference between the GC and AT pairs in DNA. It stabilizes the ds DNA by equalizing the contribution of GC- and AT-base pairs. Betaine has been broadly used to optimize multiplex and ‘long and accurate′ polymerase chain reaction (LA-PCR). The addition of 1.0-1.7 M aqueous betaine to a PCR mixture has been reported to reduce the base pair composition dependence on DNA strand melting.
Flammability and Explosibility
Non flammable
Biochem/physiol Actions
End-product of oxidative metabolism of choline, betaine is a general methyl donor, in particular in a minor pathway of methionine biosynthesis. It is used to treat homocystinuria, which is a defect in the major pathway of methionine biosynthesis.
Pharmacokinetics
Betaine decreases plasma homocysteine concentrations in homocystinuria cases caused by deficiencies or defects in cystathionine beta-synthase (CBS), 5,10-methylenetetrahydrofolate reductase (MTHFR), and cobalamin cofactor metabolism (cbl). The decrease of homocysteine is estimated to be 20-30% of pre-treatment levels. Betaine supplementation in patients with homocystinuria also improves metabolic abnormalities in cerebrospinal fluid. Reports have shown that depending on the type of homocystinuria, the therapeutic effectiveness of betaine alone may be limited, insufficient to decrease total homocysteine levels and prevent clinical symptoms. In patients with homocystinuria due to cystathionine beta-synthase (CBS) deficiency, betaine should be used when serum total homocysteine levels remain high despite dietary therapy.
Patients taking betaine for several years do not show evidence of tolerance. Also, betaine concentrations are not correlated with homocysteine concentrations. In patients with MTHFR deficiency and cbl defects, betaine may increase methionine and S-adenosyl methionine (SAM) plasma levels. Patients with CBS deficiency without a dietary restriction of methionine may accumulate excessive amounts of methionine. Clinical data shows that in patients with CBS deficiency, increased plasma methionine levels were associated with cerebral edema.
Absorption
Betaine is rapidly absorbed and distributed. In healthy volunteers (n=12) given 50 mg/kg of betaine, the Cmax, tmax and AUC0,∞ were 0.939 mmol/L, 0.90 h and 5.52 mmol?h/L, respectively. No significant changes in absorption kinetics were observed after repeated betaine administration (100 mg/kg/day for 5 days). The absolute bioavailability of betaine anhydrous has not been determined.
Metabolism
Betaine is catabolized mainly in the mitochondria of liver and kidney cells. The transmethylation of betaine via betaine homocysteine methyl transferase (BHMT) leads to the formation of dimethylglycine.
Safety
Many betaines are irritants of the eyes and skin.
Toxicity
Toxicity information regarding betaine is not readily available. Patients experiencing an overdose are at an increased risk of severe adverse effects such as cerebral edema in patients with cystathionine beta-synthase (CBS) deficiency. Symptomatic and supportive measures are recommended. In an acute toxicology study in rats, death frequently occurred at doses equal to or greater than 10,000 mg/kg. The effects of betaine on long-term carcinogenicity and fertility have not been evaluated. The following tests have not shown evidence of betaine genotoxicity: metaphase analysis of human lymphocytes, bacterial reverse mutation assay, and mouse micronucleus test.
Purification Methods
Crystallise betaine from aqueous EtOH or EtOH/Et2O. The monohydrate loses H2O above 100o. Betaine undergoes internal alkylation to methyl dimethylaminoacetate Purification of Biochemicals — Amino Acids and Peptides above its melting point. It is also prepared by treating the hydrochloride (below) with silver oxide and recrystallising from EtOH/Et2O. [Edsall J Am Chem Soc 66 1767 1943, Leifer & Lippincott J Am Chem Soc 79 5098 1957, for pK see Grob et al. Chem and Ind (London) 1222 1955, Beilstein 4 III 1127, 4 IV 2369.]
Properties of Betaine
| Melting point: | 310 °C (dec.) |
| Boiling point: | 218.95°C (rough estimate) |
| Density | 1.00 g/mL at 20 °C |
| vapor pressure | 0.05Pa at 25℃ |
| refractive index | 1.4206 (estimate) |
| FEMA | 4223 | BETAINE |
| storage temp. | 2-8°C |
| solubility | methanol: 0.1 g/mL, clear |
| pka | 1.83(at 0℃) |
| form | Crystals or Crystalline Powder |
| color | colorless |
| Odor | bland |
| Water Solubility | 160 g/100 mL |
| Sensitive | Hygroscopic |
| Merck | 14,1179 |
| BRN | 3537113 |
| Stability: | Stable. Hygroscopic. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 107-43-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Methanaminium, 1-carboxy-n,n,n-trimethyl-, hydroxide, inner salt(107-43-7) |
| EPA Substance Registry System | Betaine (107-43-7) |
Safety information for Betaine
Computed Descriptors for Betaine
| InChIKey | KWIUHFFTVRNATP-UHFFFAOYSA-N |
Abamectin manufacturer
Jeevan Chemicals and Pharmaceuticals
Unicorn Petroleum Industries Private Limited
HRV Global Life Sciences
Suvan LifeSciences (formerly Sansh Biotech Pvt Ltd)
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran




You may like
-
 Betaine 99%View Details
Betaine 99%View Details -
 Betaine 98%View Details
Betaine 98%View Details -
 Betaine CAS 107-43-7View Details
Betaine CAS 107-43-7View Details
107-43-7 -
 Betaine (Glycine Betaine) pure CAS 107-43-7View Details
Betaine (Glycine Betaine) pure CAS 107-43-7View Details
107-43-7 -
 Betaine, ≥98% CAS 107-43-7View Details
Betaine, ≥98% CAS 107-43-7View Details
107-43-7 -
 Betaine Anhydrous CAS 107-43-7View Details
Betaine Anhydrous CAS 107-43-7View Details
107-43-7 -
 Betaine 98% CAS 107-43-7View Details
Betaine 98% CAS 107-43-7View Details
107-43-7 -
 Betaine CAS 107-43-7View Details
Betaine CAS 107-43-7View Details
107-43-7