MANGANESE (III) OXIDE
Synonym(s):Manganese sesquioxide
- CAS NO.:1317-34-6
- Empirical Formula: Mn2O3
- Molecular Weight: 157.87
- MDL number: MFCD00016217
- EINECS: 215-264-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
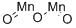
What is MANGANESE (III) OXIDE?
Chemical properties
manganese(III) oxide is a black, lustrous powder, sometimes tinged brown. Very hard.decomposes at 1080℃. Soluble in cold hydrochloric acid; not soluble in nitric acid (decomposes), hot sulfuric acid; insoluble in water. Noncombustible.

manganese(III) oxide is obtained by igniting manganese(IV) oxide or a manganese(II) salt in air at 800°C. It reacts slowly with cold dilute acids to form manganese(III) salts. Manganese(III) oxide occurs in nature as braunite (3Mn2O3.MnSiO3 ) and the monohydrate (Mn2O 3.H2O) occurs in the mineral manganite. The manganese(III) oxide crystal lattice contains Mn3+ and O2– ions. With concentrated alkalis, it undergoes disproportionation to give manganese(II) and manganese(IV) ions.
The Uses of MANGANESE (III) OXIDE
Manganese(III) oxide is used to produce ceramic magnets and semiconductors. Further, it serves as a precursor to lithium manganese oxide. In addition to this, it acts as a useful battery cathode.
Preparation
Anhydrous manganese(III) oxide is found as the mineral braunite, Mn2O3, while a hydrated form, Mn2O3.H2O, which is chemically more reactive, occurs as manganite. Manganese forms both anions and cations in oxidation state +3. Simple salts of tervalent manganese are not stable. However, complexes are more stable, the hexacyanomanganates(III) being an example: K3Mn(CN)6 is a red crystalline solid, isomorphous with potassium hexacyanoferrate(III) and slowly hydrolysed.
Manganese(III) oxide is formed as a grey solid by heating manganese(IV) oxide to 700°C for several hours:
4MnO2 ---> 2Mn203 + O2
It is converted into trimanganese tetroxide when heated above 940°C in air or reduced by hydrogen above 230°C. Hot concentrated hydrochloric acid is oxidized to chlorine by manganese(III) oxide:
Mn203 (s) + 6H + + 2e- ==2Mn2+ + 3H2O
Mn203 (s) + 6H + + 2Cl- ---> 2Mn2+ + 3H2O + Cl2
Definition
In nature as manganite, a manganese ore.
Hazard
Flammable in finely divided form. Toxic by inhalation of dust.
Flammability and Explosibility
Not classified
Safety Profile
Moderately toxic by subcutaneous and intratracheal routes. See also MANGANESE COMPOUNDS.
Properties of MANGANESE (III) OXIDE
| Melting point: | 1080°C (dec.) |
| Density | 4.5 g/mL at 25 °C(lit.) |
| solubility | insoluble in H2O |
| form | Powder |
| color | Black |
| Specific Gravity | 4.5 |
| Water Solubility | Soluble in acid and ammonium chloride. Insoluble in water, alcohol and acetone. |
| Merck | 14,5737 |
| Exposure limits | ACGIH: TWA 0.02 mg/m3; TWA 0.1 mg/m3 OSHA: Ceiling 5 mg/m3 NIOSH: IDLH 500 mg/m3; TWA 1 mg/m3; STEL 3 mg/m3 |
| CAS DataBase Reference | 1317-34-6(CAS DataBase Reference) |
| EPA Substance Registry System | Manganese(III) oxide (1317-34-6) |
Safety information for MANGANESE (III) OXIDE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for MANGANESE (III) OXIDE
| InChIKey | GEYXPJBPASPPLI-UHFFFAOYSA-N |
MANGANESE (III) OXIDE manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

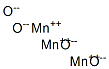
You may like
-
 Manganese (III) oxide CAS 1317-34-6View Details
Manganese (III) oxide CAS 1317-34-6View Details
1317-34-6 -
 Manganese(III) oxide CAS 1317-34-6View Details
Manganese(III) oxide CAS 1317-34-6View Details
1317-34-6 -
 Manganese (III) Oxide Nanopowder CAS 1317-34-6View Details
Manganese (III) Oxide Nanopowder CAS 1317-34-6View Details
1317-34-6 -
 Manganese(III)Oxide CAS 1317-34-6View Details
Manganese(III)Oxide CAS 1317-34-6View Details
1317-34-6 -
 Manganese (III) oxide 95.00% CAS 1317-34-6View Details
Manganese (III) oxide 95.00% CAS 1317-34-6View Details
1317-34-6 -
 Manganese(III) oxide, 99.9% CAS 1317-34-6View Details
Manganese(III) oxide, 99.9% CAS 1317-34-6View Details
1317-34-6 -
 Manganese (III) Oxide Nanopowder CAS 1317-34-6View Details
Manganese (III) Oxide Nanopowder CAS 1317-34-6View Details
1317-34-6 -
 Manganese (III) oxide 98% CAS 1317-34-6View Details
Manganese (III) oxide 98% CAS 1317-34-6View Details
1317-34-6