Cobalt oxide
Synonym(s):Cobaltous oxide
- CAS NO.:1307-96-6
- Empirical Formula: CoO
- Molecular Weight: 74.93
- MDL number: MFCD00016031
- EINECS: 215-154-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
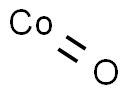
What is Cobalt oxide ?
Description
This is obtained as an olive-green powder by heating the metal in air or steam or by thermal decomposition of the hydroxide, carbonate or nitrate. It has the sodium chloride lattice and is antiferromagnetic below 292°K. When heated in oxygen above 400° the black oxide Co3O4 is obtained. This oxide is isomorphous with magnetite Fe3O4, and has tetrahedrally surrounded cobalt(II) ions and octahedrally surrounded cobalt(III) ions. Both these oxides are readily reduced to the metal by heating in hydrogen or with carbon. The reactions of CoO with silica, alumina and zinc oxide are used to form pigments in the ceramic industry.
Chemical properties
Cobalt oxide is a high-valent oxide of cobalt, with a theoretical cobalt content of 71.06% and an oxygen content of 28.94%, which is a black amorphous powder. Cobalt oxide is a kind of unstable and impossible free state compound, usually refers to the cobalt oxide are attached with a certain amount of cobalt tetraoxide when heated cobalt oxide is reduced to cobalt tetraoxide. The theoretical cobalt content of cobalt tetraoxide is 73.43% and the oxygen content is 26.57%, with the appearance of gray-black or black powder. 
The cobalt oxide used in batteries is cobalt tetraoxide, which has a spinel structure. It can be used as anode material for lithium-ion batteries and is also the main raw material for the preparation of lithium cobaltate, the cathode material for lithium-ion batteries.
Physical properties
The commercial product is usually dark grey powder, but the color may vary from olive geeen to brown depending on particle size; density 6.44 g/cm3, which also may vary between 5.7 to 6.7 g/cm3, depending on the method of preparation; melts around 1,830°C; insoluble in water; soluble in acids and alkalis.
The Uses of Cobalt oxide
In pigments for ceramics; glass coloring and decolorization; oxidation catalyst for drying oils, fast-drying paints and varnishes; preparation of cobalt-metal catalysts, Co powder for binder in sintered tungsten carbide; in semiconductors.
The Uses of Cobalt oxide
Cobalt oxide, typically 3.4-4.5%, Molybdenum oxide typically 11.5-14.5% on alumina is used in the preparation of biofuel production by using algae.
Structure and conformation
Green cobalt(II) oxide (CoO) has the rock salt (NaCl) structure. It is easily oxidized with water and oxygen to brown cobalt(III) hydroxide (Co(OH)3). At temperatures of 600℃-700℃, CoO oxidizes to the blue cobalt (II,III) oxide (Co3O4), which has a spinel structure. Black cobalt(III) oxide (Co2O3) also exists. Cobalt oxides are antiferromagnetic at low temperature: CoO (Ne′el temperature 18℃/291K) and Co3O4 (Ne′el temperature: 2233℃/40K), which is analogous to magnetite (Fe3O4), with a mixture of 12 and 13 oxidation states.
Properties of Cobalt oxide
| Melting point: | 1785 °C |
| Density | 6.45 |
| vapor pressure | 0Pa at 20℃ |
| solubility | insoluble in H2O; soluble in acid solutions |
| form | Powder |
| color | Green-brown |
| Specific Gravity | 6.45 |
| Water Solubility | insoluble |
| Sensitive | Air Sensitive |
| Merck | 14,2446 |
| Exposure limits | ACGIH: TWA 1 mg/m3 OSHA: TWA 15 mg/m3; TWA 5 mg/m3 |
| Stability: | Stability Stable, but may be moisture sensitive. |
| CAS DataBase Reference | 1307-96-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Cobalt monoxide(1307-96-6) |
| EPA Substance Registry System | Cobalt(II) oxide (1307-96-6) |
Safety information for Cobalt oxide
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H317:Sensitisation, Skin H330:Acute toxicity,inhalation H334:Sensitisation, respiratory H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for Cobalt oxide
Cobalt oxide manufacturer
Rivashaa Agrotech Biopharma Pvt. Ltd.
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 1307-96-6 Cobaltous oxide 98%View Details
1307-96-6 Cobaltous oxide 98%View Details
1307-96-6 -
 Cobalt Oxide 98%View Details
Cobalt Oxide 98%View Details -
 Cobalt(II) oxide CAS 1307-96-6View Details
Cobalt(II) oxide CAS 1307-96-6View Details
1307-96-6 -
 Cobalt(II) oxide CAS 1307-96-6View Details
Cobalt(II) oxide CAS 1307-96-6View Details
1307-96-6 -
 COBALT OXIDE 99%View Details
COBALT OXIDE 99%View Details -
 COBALT OXIDE 99%View Details
COBALT OXIDE 99%View Details -
 Cobalt(II) oxide CAS 1307-96-6View Details
Cobalt(II) oxide CAS 1307-96-6View Details
1307-96-6 -
 Cobalt(II) oxide, 99.9% CAS 1307-96-6View Details
Cobalt(II) oxide, 99.9% CAS 1307-96-6View Details
1307-96-6