Strontium nitrate
Synonym(s):Strontium nitrate
- CAS NO.:10042-76-9
- Empirical Formula: N2O6Sr
- Molecular Weight: 211.63
- MDL number: MFCD00011248
- EINECS: 233-131-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-04-23 13:52:06
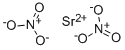
What is Strontium nitrate ?
Description
Strontium nitrate has the molecular formula of
Sr(NO3)2 and the molecular weight of 211.6327 g/mol.
Strontium nitrate is prepared by treating strontium
carbonate with nitric acid. The solution is evaporated
and crystallized:
SrCO3 +HNO3 ? Sr(NO3)2 + CO2 +H2O
Crystallization yields the tetrahydrate, Sr(NO3)2·
4H2O, which, on heating, melts at 31.3 °C in its own
waters of hydration and then decomposes >100 °C to
form the anhydrous nitrate. The anhydrate melts at
570 °C (It begins to decompose as low as 545 °C), is
soluble in water (70.3 gm/100 ml at 20 °C) and is
very slightly soluble in ethanol. These colorless crystals,
or white powder, have a density of 2.986 g/cm3
and its CAS number is 10042-76-9. It is very soluble
in water, as shown in Table 4.11 on the following page. Strontium nitrate apparently forms only the tetrahydrate
when a solution is evaporated below 80°C.
Otherwise it decomposes at higher solution temperatures
to Sr(OH)2 and nitrogen oxides. The nitric acid
concentration makes little difference in the hydrate
produced, in contrast to other alkaline earths like Ca
and Mg.
Chemical properties
Colorless cubic crystals or white powder or granules; density 2.986 g/cm3; melts at 570°C; very soluble in water, 80 g/100 mL at 18°C; very slightly soluble in ethanol.
The tetrahydrate constitutes colorless monoclinic crystals; density 2.20 g/cm3; loses all water of crystallization at 100°C; converts to strontium oxide, SrO at 1,100°C; very soluble in water, 60.4g/100 mL at 0°C, 206 g/100 mL at 100°C; soluble in liquid ammonia; very slightly soluble in ethanol and acetone.
Chemical properties
Strontium nitrate is a white crystalline solid
Physical properties
Colorless cubic crystals or white powder or granules; density 2.986 g/cm3; melts at 570°C; very soluble in water, 80 g/100 mL at 18°C; very slightly soluble in ethanol.
The tetrahydrate constitutes colorless monoclinic crystals; density 2.20 g/cm3; loses all water of crystallization at 100°C; converts to strontium oxide, SrO at 1,100°C; very soluble in water, 60.4g/100 mL at 0°C, 206 g/100 mL at 100°C; soluble in liquid ammonia; very slightly soluble in ethanol and acetone.
The Uses of Strontium nitrate
Strontium nitrate is used in pyrotechnics, for producing marine and railroad signals, and in matches.
The Uses of Strontium nitrate
Used in preparation of strontium standard solutions
The Uses of Strontium nitrate
In pyrotechnics (red fire), signal lights, marine signals, railroad flares, matches.
The Uses of Strontium nitrate
Strontium nitrate [Sr(NO3)2], because of the bright red flame it produces when burned, is used in fireworks, matches, marine signals, and so forth.
The Uses of Strontium nitrate
Industrial uses of strontium nitrate are rather limited
and have rather limited commercial value because the
corresponding calcium and barium compounds serve
the same purpose yet are cheaper. A few, however,
have found application in industry and elsewhere.
Strontium nitrate, Sr(NO3)2, and strontium chlorate,Sr(ClO3)2, are extremely volatile and impart a brilliant
crimson color to flames; they are thus used in fireworks
and similar displays.
Recent clinical studies demonstrate that when strontium
nitrate is applied to the facial skin, it significantly
reduces skincare-induced burning, stinging and facial
redness. These findings are very important to Rosacea
sufferers, who report facial burning and flushing to
topical skincare products and environmental insults
such as wind, sun, heat and cold. This is the first
compound that selectively blocks the irritationproducing
nerve endings (type C nociceptors) that
become activated when itching, burning and stinging
occur from any cause. Glycolic acid removes the upper
layer of old and dead skin cells, while encouraging the
growth of younger, smoother and evenly pigmented
looking skin. Strontium nitrate is a substance capable
of inhibiting tingling sensations and irritations associated
with peels containing glycolic acid. It reduces irritations,
inflammations, and redness caused by glycolic
acid peels.
Preparation
Strontium nitrate is prepared by treating strontium carbonate with nitric acid. The solution is evaporated and crystallized:
SrCO3 + HNO3 → Sr(NO3)2 + CO2 + H2O
Crystallization yields the tetrahydrate, Sr(NO3)2•4H2O, which on heating dehydrates to form the anhydrous nitrate.
brand name
Strotope (Bristol-Myers Squibb).
General Description
A white crystalline solid. Noncombustible but accelerates burning of combustible materials. May explode if large quantities are involved in a fire or the combustible material is finely divided. May explode under prolonged exposure to heat or fire. Toxic oxides of nitrogen are produced in fires. Used in pyrotechnics, in medicine, and to make other chemicals.
Air & Water Reactions
Soluble in water.
Reactivity Profile
Mixtures of metal/nonmetal nitrates with alkyl esters may explode, owing to the formation of alkyl nitrates; mixtures a nitrate with phosphorus, tin (II) chloride, or other reducing agents may react explosively [Bretherick 1979 p. 108-109]. Special Hazards of Combustion Products: Yields toxic gaseous oxides of nitrogen when involved in fire.
Health Hazard
Dust is irritating to skin, eyes, and respiratory system.
Flammability and Explosibility
Non flammable
Safety Profile
Moderately toxic by ingestion and intraperitoneal routes. A powerful oxidizer. When heated to decomposition it emits toxic fumes of NOx.See also NITRATES and STRONTIUM COMPOUNDS.
Potential Exposure
Strontium nitrate is used in matches, pyrotechnics, marine signals; and railroad flares.
Shipping
UN1507 Strontium nitrate, Hazard Class: 5.1; Labels: 5.1-Oxidizer.
Purification Methods
Crystallise it from hot water (0.5mL/g) by cooling to 0o.
Incompatibilities
A strong oxidizer. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Violent reaction with reducing agents; combustibles, organics, or other readily oxidizable materials. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.
Properties of Strontium nitrate
| Melting point: | 570 °C (lit.) |
| Boiling point: | 645 °C |
| Density | 2.99 |
| storage temp. | Store at +5°C to +30°C. |
| solubility | 660g/l |
| form | Solid |
| color | White |
| Specific Gravity | 2.99 |
| PH Range | 5.0 - 7.0 |
| Odor | Odorless |
| PH | 5-7 (50g/l, H2O, 20℃) |
| Water Solubility | 660 g/L (20 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,8845 |
| Stability: | Strong oxidizer - contact with combustible material may cause fire. Incompatible with strong reducing agents, combustible material. |
| CAS DataBase Reference | 10042-76-9(CAS DataBase Reference) |
| EPA Substance Registry System | Strontium nitrate (10042-76-9) |
Safety information for Strontium nitrate
| Signal word | Danger |
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H271:Oxidising liquids;Oxidising solids H318:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P220:Keep/Store away from clothing/…/combustible materials. P280:Wear protective gloves/protective clothing/eye protection/face protection. P283:Wear fire/flame resistant/retardant clothing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P306+P360:IF ON CLOTHING: Rinse Immediately contaminated CLOTHING and SKIN with plenty of water before removing clothes. |
Computed Descriptors for Strontium nitrate
| InChIKey | DHEQXMRUPNDRPG-UHFFFAOYSA-N |
Abamectin manufacturer
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

You may like
-
 Strontium nitrate CAS 10042-76-9View Details
Strontium nitrate CAS 10042-76-9View Details
10042-76-9 -
 Strontium nitrate CAS 10042-76-9View Details
Strontium nitrate CAS 10042-76-9View Details
10042-76-9 -
 Strontium nitrate, Anhydrous CAS 10042-76-9View Details
Strontium nitrate, Anhydrous CAS 10042-76-9View Details
10042-76-9 -
 Strontium nitrate, Anhydrous CAS 10042-76-9View Details
Strontium nitrate, Anhydrous CAS 10042-76-9View Details
10042-76-9 -
 Strontium nitrate, Anhydrous CAS 10042-76-9View Details
Strontium nitrate, Anhydrous CAS 10042-76-9View Details
10042-76-9 -
 Strontium nitrate, Anhydrous CAS 10042-76-9View Details
Strontium nitrate, Anhydrous CAS 10042-76-9View Details
10042-76-9 -
 Strontium nitrate CAS 10042-76-9View Details
Strontium nitrate CAS 10042-76-9View Details
10042-76-9 -
 Strontium nitrate CAS 10042-76-9View Details
Strontium nitrate CAS 10042-76-9View Details
10042-76-9