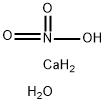
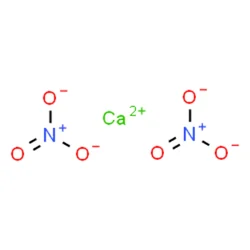
Calcium nitrate
- CAS NO.:10124-37-5
- Empirical Formula: CaN2O6
- Molecular Weight: 164.09
- MDL number: MFCD00010899
- EINECS: 233-332-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
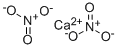
What is Calcium nitrate?
Description
Calcium nitrate, with the molecular formula Ca(NO?)?, is prepared by reacting nitric acid with calcium carbonate or calcium sulfide.
The anhydrous form (CAS: 10124-37-5) is a hygroscopic, white cubic crystal with a density of 2.504 g/cm3 and a melting point of 561°C. It is highly soluble in water, as well as in alcohols and acetone. For instance, its aqueous solubility is 121.2 g/100 mL at 20°C and increases to 271.2 g/100 mL at 40°C.
Upon evaporation of its aqueous solution, calcium nitrate crystallizes as a tetrahydrate, Ca(NO?)?·4H?O (CAS: 13477-34-4). This hydrate melts in its own water of crystallization at 42.7°C and loses all four water molecules when heated to 132°C. Upon further heating, it decomposes to yield calcium oxide and nitrogen oxides, a property common to other alkaline earth metal nitrates.
The specific hydrate formed is dependent on the concentration of nitric acid used during crystallization, with a known trihydrate also existing. A monohydrate has not been identified.
Chemical properties
White, deliquescent mass. Soluble in water, alcohol, and acetone.
Physical properties
White cubic crystal; hygroscopic; density 2.50g/cm3; melts at 561°C; highly soluble in water; also dissolves in alcohols and acetone.
The Uses of Calcium nitrate
Calcium nitrate's primary application is as a nitrogen and calcium source in fertilizers. It is also the principal component of the mineral nitrocalcite, which forms as an efflorescence where manure contacts limestone in dry environments like stables or caverns. Beyond agriculture, calcium nitrate serves multiple industrial roles. It is used as a component in explosives, matches, and pyrotechnics. Its applications extend to the manufacturing of incandescent mantles and as a corrosion-inhibiting additive for diesel fuel. In manufacturing processes, it acts as a flocculant for rubber latex and is used in the production of refrigerants. Additional uses include its employment in oil exploratory wells and for wastewater treatment. It is also a raw material for producing other nitrate compounds. As a strong oxidizing agent, calcium nitrate can form explosive mixtures when combined with organic materials or other oxidizable compounds. Due to its thermal properties, it has also been evaluated for use in heat storage devices. However, its utility as an oxidizer is limited by its hygroscopic nature, which causes it to be highly deliquescent.
The Uses of Calcium nitrate
In explosives, fertilizers, matches, pyrotechnics; manufacture of incandescent mantles, radio tubes, HNO3; corrosion inhibitor in diesel fuels.
The Uses of Calcium nitrate
Calcium nitrate [Ca(NO3)2] is known as Norwegian saltpeter. It is a strong oxidizer (because of the NO3) that is flammable in the presence of organic materials (such as hands). It explodes when given a hard shock. It is used in fireworks, matches, and fertilizers.
Definition
ChEBI: Inorganic nitrate salt of calcium.
Definition
calcium nitrate: A white deliquescentcompound, Ca(NO3)2, that isvery soluble in water; cubic; r.d. 2.50;m.p. 561°C. It can be prepared byneutralizing nitric acid with calciumcarbonate and crystallizing it fromsolution as the tetrahydrateCa(NO3)2.4H2O, which exists in twomonoclinic crystalline forms (α, r.d.1.9; β, r.d. 1.82). There is also a trihydrate,Ca(NO3)2.3H2O. The anhydroussalt can be obtained from the hydrateby heating but it decomposeson strong heating to give the oxide,nitrogen dioxide, and oxygen. Calciumnitrate is sometimes used as anitrogenous fertilizer.
Preparation
Calcium nitrate may be prepared by the reaction of nitric acid with calcium carbonate or calcium sulfide:
CaCO3 + 2HNO3 →Ca(NO3)2 + CO + H2O
CaS + 2HNO3 →Ca(NO3)2 + H2S
General Description
White to light gray granular solid. May be either the anhydrous compound or the tetrahydrate. Used in fertilizers, explosives and pyrotechnics.
Air & Water Reactions
Deliquescent. Water soluble.
Reactivity Profile
An oxidizing agent. Noncombustible but accelerates the burning of combustible materials. If large quantities are involved in the fire or the combustible material is finely divided an explosion may result. Prolonged exposure to fire or heat may result in an explosion. May explode if shocked or heated [Hawley]. Heating causes release of toxic oxides of nitrogen. Mixtures with alkyl esters may explode owing to the formation of alkyl nitrates; mixtures with phosphorus, tin(II) chloride, or other reducing agents may react explosively [Bretherick 1979 p. 108-109].
Hazard
Strong oxidizer, dangerous fire risk in con- tact with organic materials, may explode if shocked or heated.
Health Hazard
Dust causes mild irritation of eyes.
Flammability and Explosibility
Non flammable
Agricultural Uses
Calcium nitrate, also known as lime nitrate or Norwegian saltpeter, was the first synthetic nitrogen fertilizer to be commercialized. As a fertilizer, it provides 15.5% nitrogen and 21% calcium. It is effective for controlling blossom-end rot in tomatoes and is a major nitrogen source in European agriculture. Furthermore, it can improve the physical properties of acidic and saline soils by displacing sodium with calcium and having a non-acidifying effect. However, the compound is extremely hygroscopic, readily absorbing significant amounts of water. This property complicates handling and requires manufacturing in climate-controlled facilities and packaging in sealed, moisture-proof bags. To address this, it is often produced in a prilled (granulated) form, which is suitable for bulk mixing, while the powdered form is used for foliar sprays. Beyond its primary use in agriculture, calcium nitrate is also utilized in the manufacturing of explosives, pyrotechnics, and in various inorganic chemical processes.
Agricultural Uses
Nitrocalcite is another name for calcium nitrate. It was
the first chemical nitrogenous fertilizer to be marketed.
Nitro-carbonic process for calcium carbonate
In manufacturing nitrophosphate fertilizers by Odda
process, calcium nitrate is left behind in the solution. It is
highly hygroscopic and is thus very inconvenient to use
as a fertilizer. It has, therefore, to be removed or
modified to some other form, which is done by the nitrocarbonic
process. The process converts calcium nitrate to
calcium carbonate by injecting carbon dioxide into the
calcium nitrate solution.
Safety Profile
A poison by ingestion. An irritant. A strong oxidant. Forms powerfully explosive mixtures with aluminum + ammonium nitrate + formamide + water, ammonium nitrate + hydrocarbon oils, ammonium nitrate + water-soluble fuels, and organic materials. When heated to decomposition it emits toxic fumes of NOx. See also NITRATES and CALCIUM COMPOUNDS.
Potential Exposure
It is used to make explosives, fertilizers, matches, fireworks, and other industrial products.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure, beginrescue breathing (using universal precautions, includingresuscitation mask) if breathing has stopped and CPR if heartaction has stopped. Transfer promptly to a medical facility.When this chemical has been swallowed, get medical attention. Give large quantities of water and induce vomiting. Donot make an unconscious person vomit.Note to physician: Treat for methemoglobinemia.Spectrophotometry may be required for precise determination of levels of methemoglobin in urine.
Storage
lor Code—Yellow: Reactive Hazard; Store in alocation separate from other materials, especially flam?mables and combustibles. Prior to working with Calcium540 Calcium nitratenitrate you should be trained on its proper handling andstorage. Store in tightly closed containers in a cool, wellventilated area away from flammables (such as fuel) orcombustibles (such as wood, paper, and oil). Calciumnitrate may explode if shocked or heated. See OSHAStandard 1910.104 and NFPA 43A Code for the Storage ofLiquid and Solid Oxidizers for detailed handling and storageregulations.
Shipping
Calcium nitrate must be labeled“OXIDIZER.” It falls in Hazard Class 5.1 and PackingGroup III.
Incompatibilities
A strong oxidizer. Incompatible withcombustible materials, reducing agents, organics and otheroxidizable materials, chemically active metals, aluminumnitrate, ammonium nitrate.
Properties of Calcium nitrate
| Melting point: | 561°C |
| Boiling point: | 130-140 °C |
| Density | 2.36 |
| solubility | soluble in ethanol, methanol, acetone |
| form | Liquid |
| color | white cubic crystals, crystalline; hygroscopic |
| Water Solubility | Soluble |
| CAS DataBase Reference | 10124-37-5(CAS DataBase Reference) |
| EPA Substance Registry System | Calcium nitrate (10124-37-5) |
Safety information for Calcium nitrate
Computed Descriptors for Calcium nitrate
Calcium nitrate manufacturer
Ekhande Agro Fertilizers Private Limited
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 Calcium Nitrate Granular FertilizerView Details
Calcium Nitrate Granular FertilizerView Details
10124-37-5 -
 Granules Calcium Nitrate, 25 KgView Details
Granules Calcium Nitrate, 25 KgView Details
10124-37-5 -
 Granules Calcium Nitrate granular, fertilizer, 25Kg BagView Details
Granules Calcium Nitrate granular, fertilizer, 25Kg BagView Details
10124-37-5 -
 Shiv Chemicals White Calcium Nitrate Granular, Packaging Type: Hdpe Bags, Packaging Size: 25 KgView Details
Shiv Chemicals White Calcium Nitrate Granular, Packaging Type: Hdpe Bags, Packaging Size: 25 KgView Details
10124-37-5 -
 Calcium NitrateView Details
Calcium NitrateView Details
10124-37-5 -
 calcium nitrateView Details
calcium nitrateView Details
10124-37-5 -
 50 Kg White Calcium Nitrate Fertilizer, BagsView Details
50 Kg White Calcium Nitrate Fertilizer, BagsView Details
10124-37-5 -
 Chemical Grade Calcium Nitrate (N-15.5%, Ca-17%, B-0.25%), Packaging Size: 25 kg, VegetablesView Details
Chemical Grade Calcium Nitrate (N-15.5%, Ca-17%, B-0.25%), Packaging Size: 25 kg, VegetablesView Details
10124-37-5
