Stannous chloride
- CAS NO.:7772-99-8
- Empirical Formula: Cl2Sn
- Molecular Weight: 189.62
- MDL number: MFCD00011241
- EINECS: 231-868-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:56
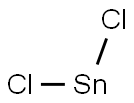
What is Stannous chloride?
Chemical properties
Also known as tin chloride, tin crystals, tin dichloride and tin salts, SnCl2 is white crystals, soluble in water, alcohol, and alkalies, oxidized in air to the oxychloride, that melt at 247°C. Used as a chemical intermediate, reducing agent, and ink-stain remover, and for silvering mirrors.
Chemical properties
Stannous chloride is a white crystalline solid.
Physical properties
White orthogonal crystal; density 3.90 g/cm3; melts at 247°C; vaporizes at 623°C; vapor pressure 1 torr at 316°C, 5 torr at 366°C and 20 torr at 420°C; soluble in water, ethanol, acetone and ether; insoluble in xylene and mineral spirits.
TIN(II) CHLORIDE 937The dihydrate, SnCl2•2H2O, is a white monoclinic crystalline substance; density 2.71 g/cm3; absorbs oxygen from air forming an oxychloride; melts at 37°C on rapid heating; decomposes on strong heating; very soluble in water; forms an insoluble basic salt with excess water; very soluble in hydrochloric acid; soluble in caustic soda solution, ethanol and ethyl acetate.
The Uses of Stannous chloride
Stannous Chloride is an antioxidant and preservative that exists as white or colorless crystals, being very soluble in water. it reacts read- ily with oxygen, preventing its combination with chemicals and foods which would otherwise result in discoloration and undesirable odors. it is used for color retention in asparagus at less than 20 ppm. it is also used in carbonated drinks.
The Uses of Stannous chloride
Synthesis of tin(IV) octaethylcorroles was accomplished with this reagent. These new compounds exhibit reversible oxidation only at the conjugated ring system, not at the metal center.1
The Uses of Stannous chloride
Tin (II) chloride is a strong reducing agent and is used in many industrial processes, such as manufacturing dyes, phosphors, and polymers. The compound is a major ingredient in acid tin plating baths. Other uses are a mordant in dyeing; an additive to lubricating oil to prevent sludging; a stablizier for perfume in soaps; in removing ink stains; a sensitizing agent for glass, paper, and plastics; and a soldering flux. Tin(II) chloride is used for preparing a number of tin(II) salts. It is a catalyst in many organic reactions. It is a common laboratory reagent.
What are the applications of Application
Tin(II) chloride is a tin preparation used as a laboratory reductant
Indications
Stannous chloride is a component of technetium-99m agents indicated for imaging of the skeleton for areas of altered osteogenesis or in the detection of infarcted heart tissue .
Background
Stannous chloride is used as a source of tin in radiopharmaceutical kits. Tin reduces technetium-99m, the active radiological agent, allowing it to form a complex with phosphate-containing moeities . These complexes localize primarily in bone (40-50%) and infracted myocardium (0.01-0.02%/g of tissue) allowing for imaging of areas of altered osteogenesis or necrotic heart tissue .
Definition
ChEBI: An inorganic chloride that has formula Cl2Sn.
Preparation
Tin(II) chloride is prepared by dissolving tin in hydrochloric acid followed by evaporation of the solution and crystallization.
General Description
Crystalline mass or flaky solid with a fatty appearance. Density 3.95 g / cm3. Melting point 247°C. Burns, but may be difficult to ignite. Toxic by ingestion. Irritates skin and eyes. Used in the manufacture of dyes, pharmaceuticals and as a tanning agent.
Air & Water Reactions
Water soluble.
Reactivity Profile
STANNOUS CHLORIDE is a powerful reducing agent. Can react violently with oxidizing agents. Undergoes flaming reaction with bromine trifluoride [Mellor 2 Supp. 1:164 1956]. Catalyzes the exothermic rearrangement and polymerization of ethylene oxide [J. Soc. Chem. Ind. 68:179 1949]. Mixtures with calcium carbide can be ignited with a match, and the reaction proceeds with incandescence [Mellor 7:430 1946-47]. Reacts with hydrazine hydrate to give stannous dihydrazine chloride which decomposes explosively when heated [Mellor 7:430 1946-47]. Undergoes a strongly exothermic reaction with aqueous solutions of hydrogen peroxide having concentration exceeding 3%) [Chem. & Ind., 1949, 657].
Hazard
Irritant to skin, use in foods restricted to 0.0015%, as tin.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Pharmacokinetics
Stannous chloride itself is not known to produce a pharmacological effect at the dosages used clinically.
Safety Profile
Poison by ingestion, intraperitoneal, intravenous, and subcutaneous routes. Experimental reproductive effects. Human mutation data reported. Potentially explosive reaction with metal nitrates. Violent reactions with hydrogen peroxide, ethylene oxide, hydrazine hydrate, nitrates, K, Na. Ignition on contact with bromine trifluoride. A vigorous reaction with calcium acetylide is initiated by flame. When heated to decomposition it emits toxic fumes of Cl-. See also TIN COMPOUNDS.
Potential Exposure
Stannous chloride is used as a dye, pigment, and printing ink; in making chemicals; chemical preservatives; food additives; polymers, textiles, glass, silvering mirrors.
Metabolism
Not Available
Shipping
UN3260 Corrosive solid, acidic, inorganic, n.o.s., Hazard class: 8; Labels: 8-Corrosive material, Technical Name Required.
Purification Methods
Analytical reagent grade stannous chloride dihydrate is dehydrated by adding it slowly to vigorously stirred, redistilled acetic anhydride (120g salt per 100g of anhydride) in a fume cupboard. After ca an hour, the anhydrous SnCl2 is filtered on to a sintered-glass or Büchner funnel, washed free from acetic acid with dry Et2O (2 x 30mL), and dried under vacuum. It is stored in a sealed container. [Stephen J Chem Soc 2786 1930, Williams Org Synth Coll Vol III 627 1955.]
Incompatibilities
A strong reducing agent. Reacts violently with oxidants. Reacts violently with bromine trifluoride; potassium, hydrazine hydrate, sodium, sodium peroxide; ethylene oxide; and nitrates. Keep away from moisture, sources of oxygen, and combustible materials.
Properties of Stannous chloride
| Melting point: | 246 °C(lit.) |
| Boiling point: | 652 °C(lit.) |
| Density | 3.95 |
| vapor pressure | 0Pa at 20℃ |
| Flash point: | 652°C |
| storage temp. | Store below +30°C. |
| solubility | H2O: soluble |
| form | powder |
| Specific Gravity | 3.95 |
| color | White |
| PH | 2.0 (100g/l, H2O, 20℃) |
| Water Solubility | Soluble in water, alkalies, alcohol, methyl ethyl ketone, methyl acetate and acetone. |
| Sensitive | Air Sensitive & Hygroscopic |
| Merck | 14,8783 |
| Exposure limits | ACGIH: TWA 2 mg/m3 NIOSH: IDLH 100 mg/m3; TWA 2 mg/m3 |
| Stability: | Stable, but moisture sensitive. Incompatible with strong bases, strong oxidizing agents, reactive metals, hydrogen peroxide, water. |
| CAS DataBase Reference | 7772-99-8(CAS DataBase Reference) |
| EPA Substance Registry System | Tin dichloride (7772-99-8) |
Safety information for Stannous chloride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H290:Corrosive to Metals H314:Skin corrosion/irritation H317:Sensitisation, Skin H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H373:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Stannous chloride
| InChIKey | AXZWODMDQAVCJE-UHFFFAOYSA-L |
Stannous chloride manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 TIN CHLORIDE 99%View Details
TIN CHLORIDE 99%View Details -
 Tin(II) chloride hydrate CAS 7772-99-8View Details
Tin(II) chloride hydrate CAS 7772-99-8View Details
7772-99-8 -
 Tin(II) Chloride, Ampouled under argon CAS 7772-99-8View Details
Tin(II) Chloride, Ampouled under argon CAS 7772-99-8View Details
7772-99-8 -
 Tin(II) Chloride, Ampouled under argon CAS 7772-99-8View Details
Tin(II) Chloride, Ampouled under argon CAS 7772-99-8View Details
7772-99-8 -
 Tin(II) chloride, anhydrous CAS 7772-99-8View Details
Tin(II) chloride, anhydrous CAS 7772-99-8View Details
7772-99-8 -
 Tin(II) chloride, anhydrous CAS 7772-99-8View Details
Tin(II) chloride, anhydrous CAS 7772-99-8View Details
7772-99-8 -
 Tin(II) chloride, anhydrous CAS 7772-99-8View Details
Tin(II) chloride, anhydrous CAS 7772-99-8View Details
7772-99-8 -
 Stannous Chloride, 50 Kg BagView Details
Stannous Chloride, 50 Kg BagView Details
10025-69-1
