BARIUM BROMIDE
Synonym(s):Barium dibromide
- CAS NO.:10553-31-8
- Empirical Formula: BaBr2
- Molecular Weight: 297.14
- MDL number: MFCD00003444
- EINECS: 234-140-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
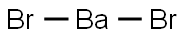
What is BARIUM BROMIDE?
Description
Barium bromide has the molecular formula of BaBr2.
Like barium chloride, it is soluble in water but is toxic if
ingested. BaBr2 crystallizes in a PbCl2 motif, as white
orthorhombic crystals that are deliquescent. In aqueous
solution, BaBr2 behaves as a simple salt.
Barium bromide can be crystallized from its solution
as a dihydrate, BaBr2·2H2O (molecular weight: 333.19),
which forms the anhydrate by heating to 120°C. It
dissolves in its own water-of-hydration at 75°C before
decomposing to the anhydrate.
Chemical properties
White Crysatalline Powder
Physical properties
White orthorhombic crystal; density of anhydrous BaCl2 4.78 g/cm3, and dihydrate BaCl2 ? 2H2O 3.58 g/cm3; melts at 857°C; vaporizes at 1,835°C; readily dissolves in water (92.2 g/100 g water at 0°C).
The Uses of BARIUM BROMIDE
Barium bromide is a precursor to chemicals used in photography as well as to form other bromides.Historically, barium bromide was used to purify radium in a process of fractional crystallization devised by Marie Curie. Since radium precipitates preferentially in a solution of barium bromide, the ratio of radium to barium in the precipitate was found to be higher than the ratio in the solution.
The Uses of BARIUM BROMIDE
In the manufacture of other bromides; in the preparation of phosphors.
The Uses of BARIUM BROMIDE
Barium bromide, anhydrous is used as the chemical intermediate, and the spice cosmetics chemical additive. It is also used as pharmaceutical intermediate.
Preparation
Barium bromide can be prepared from barium
sulfide or barium carbonate by reaction with hydrobromic
acid to give hydrated barium bromide:
BaS+HBr→BaBr2+H2S
BaCO3+HBr→BaBr2+CO2+H2O
Hazard
Ingestion of the salt or its aqueous solution can produce severe poisoning.
Properties of BARIUM BROMIDE
| Melting point: | 850 °C |
| Boiling point: | 1835°C |
| Density | 4,781 g/cm3 |
| form | beads |
| color | White |
| Specific Gravity | 4.781 |
| Water Solubility | Soluble in water |
| Sensitive | Hygroscopic |
| Merck | 14,968 |
| Exposure limits | ACGIH: TWA 0.5 mg/m3 NIOSH: IDLH 50 mg/m3; TWA 0.5 mg/m3 |
| Stability: | Stable. |
| CAS DataBase Reference | 10553-31-8(CAS DataBase Reference) |
| EPA Substance Registry System | Barium bromide (BaBr2) (10553-31-8) |
Safety information for BARIUM BROMIDE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. |
Computed Descriptors for BARIUM BROMIDE
BARIUM BROMIDE manufacturer
JSK Chemicals
A.B.ENTERPRISES
Axiom Chemicals Pvt Ltd (Axiom Corporation)
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
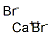
You may like
-
 BARIUM BROMIDE 98%View Details
BARIUM BROMIDE 98%View Details -
 BARIUM BROMIDE 99%View Details
BARIUM BROMIDE 99%View Details -
 Barium bromide, Ultra dry CAS 10553-31-8View Details
Barium bromide, Ultra dry CAS 10553-31-8View Details
10553-31-8 -
 Barium bromide, Ultra dry CAS 10553-31-8View Details
Barium bromide, Ultra dry CAS 10553-31-8View Details
10553-31-8 -
 Barium bromide, Anhydrous CAS 10553-31-8View Details
Barium bromide, Anhydrous CAS 10553-31-8View Details
10553-31-8 -
 Barium bromide, Anhydrous CAS 10553-31-8View Details
Barium bromide, Anhydrous CAS 10553-31-8View Details
10553-31-8 -
 Barium Bromide CASView Details
Barium Bromide CASView Details -
 Barium bromide CAS 10553-31-8View Details
Barium bromide CAS 10553-31-8View Details
10553-31-8