SPIRAMYCIN I
- CAS NO.:24916-50-5
- Empirical Formula: C43H74N2O14
- Molecular Weight: 843.05
- MDL number: MFCD00242797
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-18 11:31:17
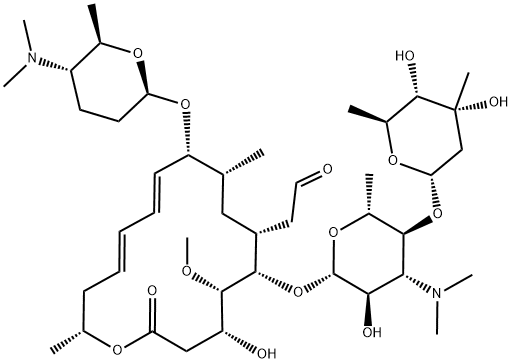
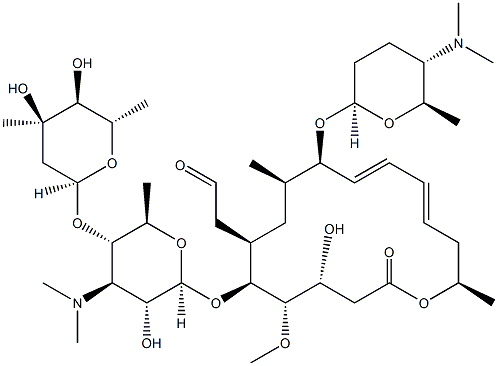
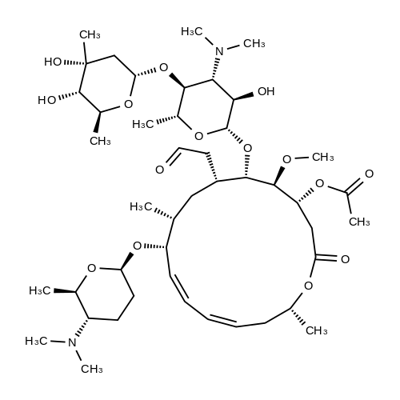
What is SPIRAMYCIN I?
Absorption
The extent of absorption of Spiramycin was shown to be incomplete. Oral bioavailability ranges from 30-39%. Spiramycin has slower rate of absorption than Erythromycin. It has a high pKa (7.9) which could be a result of high degree of ionization in acidic medium of the stomach.
Toxicity
Cardiac toxicity, specifically QT prolongation (irregular heartbeat; recurrent fainting). Cholestatic hepatitis (abdominal pain; nausea; vomiting; yellow eyes or skin). Gastrointestinal toxicity, specifically acute colitis (abdominal pain and tenderness; bloody stools; fever). Intestinal injury (abdominal pain and tenderness). Ulcerated esophagitis (chest pain; heartburn).
Chemical properties
Crystalline Solid
The Uses of SPIRAMYCIN I
Antibiotic substance classified in the erythromycin-carbomycin group
The Uses of SPIRAMYCIN I
Antibiotic substance classified in the erythromycin-carbomycin group.
What are the applications of Application
Spiramycin I is an antibiotic from the erythromycin-carbomycin group
Indications
Macrolide antibiotic for treatment of various infections.
Background
Spiramycin is a primarily bacteriostatic macrolide antimicrobial agent with activity against Gram-positive cocci and rods, Gram-negative cocci and also Legionellae, mycoplasmas, chlamydiae, some types of spirochetes, Toxoplasma gondii and Cryptosporidium. Spiramycin is a 16-membered ring macrolide discovered in 1952 as a product of Streptomyces ambofaciens that has been available in oral formulations since 1955, and parenteral formulations since 1987. Resistant organisms include Enterobacteria, pseudomonads, and moulds.
Definition
ChEBI: A macrolide antibiotic produced by various Streptomyces species that is used to treat toxoplasmosis and various other infections of soft tissues.
Pharmacokinetics
The absolute bioavailability of oral spiramycin is generally within the range of 30 to 40%. After a 1 g oral dose, the maximum serum drug concentration was found to be within the range 0.4 to 1.4 mg/L.
Metabolism
Spiramycin is less metabolised than some of the other macrolides. Metabolism has not been well studied. It is mainly done in the liver to the active metabolites.
Properties of SPIRAMYCIN I
| Melting point: | 134-1370C |
| alpha | D20 -96° |
| Boiling point: | 913.7±65.0 °C(Predicted) |
| Density | 1.21±0.1 g/cm3(Predicted) |
| storage temp. | Inert atmosphere,Store in freezer, under -20°C |
| solubility | DMSO (Slightly), Ethanol (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 13.06±0.70(Predicted) |
| color | White to Off-White |
| Stability: | Moisture Sensitive, Temperature Sensitive |
Safety information for SPIRAMYCIN I
Computed Descriptors for SPIRAMYCIN I
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1