Spiramycin
Synonym(s):Formacidine
- CAS NO.:8025-81-8
- Empirical Formula: C43H74N2O14
- Molecular Weight: 843.05
- MDL number: MFCD01314545
- EINECS: 232-429-6
- Update Date: 2025-12-16 16:15:04
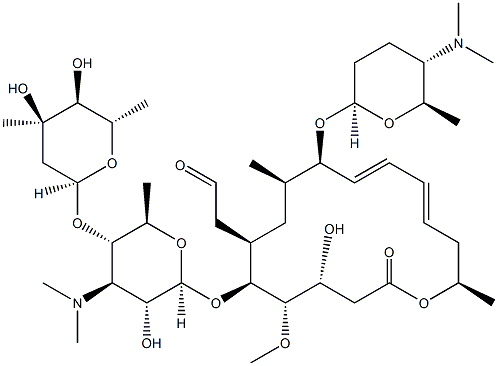
What is Spiramycin?
Description
Spiramycin was found in the culture broth of Streptomyces ambofaciens by Rhone Poulenc in 1954 and its acetate was synthesized by Kyowa Hakko Kogyo Co. in 1965. This antibiotic consists of three closely related macrolide components, I, II, and III, whose ratio is 2 : 1 : 1. Spiramycin shows almost the same antimicrobial spectrum and activity as the other macrolides, but its acetate, acetylspiramycin, has much better pharmacokinetic properties and activity in vivo.
Chemical properties
An antibiotic substance.
Originator
Rovamycine,Specia,France,1972
The Uses of Spiramycin
Spiramycin I (foromacidin A) is the major analogue of a complex of 16-membered macrocyclic lactones produced by S. ambofaciens and S. spiramyceticus that have broad spectrum antibiotic activity. Spiramycins are unusual among the macrocyclic lactones in that they contain two basic sugars. Spiramycin complex has been used in both human and animal health but its use has not been widespread.
The Uses of Spiramycin
antibacterial
The Uses of Spiramycin
vitamin A
Manufacturing Process
The process for producing spiramycin comprises inoculating an aqueous nutrient medium with a culture of the NRRL No. 2420, allowing aerobic fermentation to take place and separating from the culture medium the spiramycin thus formed. The culture medium also contains the antibiotic substance known as Congocidin which, however, does not possess the same useful properties as spiramycin and which can be isolated in crystalline form. The separation of the two antibiotic substances is readily achieved.
brand name
Rovamycin (Rhone-Poulenc Rorer).
Therapeutic Function
Antibacterial
Antimicrobial activity
Enterobacteriaceae are resistant. Spiramycin is also active against anaerobic species: Actinomyces israelii (MIC 2–4 mg/L), Cl. perfringens (MIC 2–8 mg/L) and Bacteroides spp. (MIC 4–14 mg/L). It is also active against Tox. gondii.
Hazard
Poison; moderately toxic; teratogen; muta- gen; can cause hypermotility, diarrhea, nausea, or vomiting.
Pharmaceutical Applications
A fermentation product of Streptomyces ambofaciens, composed of several closely related compounds. Spiramycin 1 is the major component (c. 63%); spiramycins 2 and 3 are the acetate and monopropionate esters, respectively. It is available for oral administration and as spiramycin adipate for intravenous infusion. Spiramycins are relatively stable in acid conditions. A derivative, acetylspiramycin, is available in Japan.
Pharmacokinetics
Oral absorption: Variable
Cmax 1 g oral: 2.8 mg/L after 2 h
Plasma half-life:4–8h
Volume of distribution :383 L
Plasma protein binding :15%
In healthy volunteers given 2 g orally followed by 1 g every 6 h, peak plasma levels were 1.0–6.7 mg/L. After 1 g orally the AUC was 10.8 mg.h/L, with an apparent elimination halflife of 2.8 h. It is widely distributed in the tissues. It does not reach the CSF. Levels 12 h after a dose of 1 g were 0.25 mg/L in serum, 5.3 mg/L in bone and 6.9 mg/L in pus. Levels of 10.6 mg/L have been found 4 h after dosing in saliva, and concentrations at least equal to those in the serum are seen in bronchial secretions. A concentration of 27 mg/g was found in prostate tissues after repeated dosage. Only 5–15% is recovered from the urine. Most is metabolized, but significant quantities are eliminated via the bile, in which concentrations up to 40 times those in the serum may be found.
Side Effects
Spiramycin is generally well tolerated, the most common adverse reactions being gastrointestinal disturbances, notably abdominal pain, nausea and vomiting, rashes and sensitization following contact.
Properties of Spiramycin
| Melting point: | 126-128 °C |
| Boiling point: | 914℃ |
| alpha | D20 -80° (methanol) |
| refractive index | 81 ° (C=2, 10% AcOH) |
| Flash point: | >110°(230°F) |
| storage temp. | 2-8°C |
| solubility | ethanol: 50 mg/mL, clear to slightly hazy, light-yellow |
| form | powder |
| color | white to light yellow |
| optical activity | [α]/D -85 to -80° in water (Specific rotation (dry basis)) |
| Water Solubility | Soluble in methanol. Slightly soluble in water |
| Merck | 14,8752 |
| CAS DataBase Reference | 8025-81-8 |
Safety information for Spiramycin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H303:Acute toxicity,oral |
| Precautionary Statement Codes |
P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P403:Store in a well-ventilated place. |
Computed Descriptors for Spiramycin
Spiramycin manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
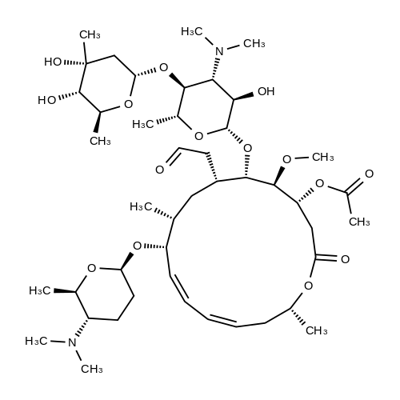

![Spiramycin, 4,4'-methylenebis[3-hydroxy-2-naphthalenecarboxylate] (ester)](https://img.chemicalbook.in/)


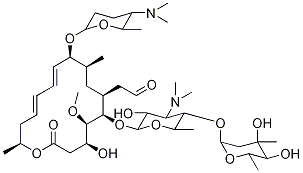

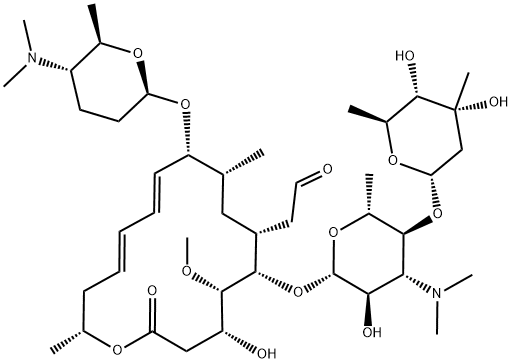
You may like
-
 Spiramycin CAS 8025-81-8View Details
Spiramycin CAS 8025-81-8View Details
8025-81-8 -
 Spiramycin 90.00% CAS 8025-81-8View Details
Spiramycin 90.00% CAS 8025-81-8View Details
8025-81-8 -
 Spiramycin from Streptomyces sp. CAS 8025-81-8View Details
Spiramycin from Streptomyces sp. CAS 8025-81-8View Details
8025-81-8 -
 Spiracin Spiramycin TabletsView Details
Spiracin Spiramycin TabletsView Details
8025-81-8 -
 Spiracin Spiramycin TabletsView Details
Spiracin Spiramycin TabletsView Details
8025-81-8 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
