Sodium thiomethoxide
Synonym(s):Methanethiol sodium salt;Sodium methanethiolate;Sodium methanethiolate solution;Sodium thiomethoxide;Sodium thiomethoxide solution
- CAS NO.:5188-07-8
- Empirical Formula: CH3NaS
- Molecular Weight: 70.09
- MDL number: MFCD00174316
- EINECS: 225-969-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
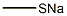
What is Sodium thiomethoxide?
Description
Sodium methanethiolate or sodium thiomethoxide (CH3SNa, MeSNa) is the sodium conjugate base of methanethiol. This compound is commercially available as a white solid. Sodium Methanethiolate is used as a nucleophile in organic synthesis. This compound is also being used as a reagent to synthesize thiol-based suberoylanilide hydroxamic acid (SAHA) analogues, compounds that act as potent histone deacetylase inhibitors. It reacts with trifluoroacetic acid and water to produce the active form of sodium trifluoroacetate. The reaction mechanism is likely due to the formation of a bicyclic heterocycle that has been shown to be effective against a number of bacteria.
Chemical properties
Very faint yellowish red clear liquid
The Uses of Sodium thiomethoxide
Sodium thiomethoxide is a strong nucleophile used for the synthesis of methyl aryl sulfides from halo-arenes. Alkanethiolates are efficient reagents for SN2 dealkylation of esters and aryl ethers.
The Uses of Sodium thiomethoxide

A mixture of the SM (3 g, 9.7 mmol) and aq NaSMe (18 mL, 38.9 mmol, 21% w/v) in DMF (75 mL) was heated to 60 C for 2 h, after which time it was cooled to RT, poured into H2O (150 mL), and acidified with 1.5N HCl. The resulting solids were filtered to provide the product as an off-white solid. [2.5 g]
The Uses of Sodium thiomethoxide
Used to prepare a C-21 thioether of methyl 16-prednisolonecarboxylate by mesylate displacement.
What are the applications of Application
Sodium Methanethiolate is A strong nucleophile
Reactivity Profile
Sodium thiomethoxide is a powerful nucleophile that can be used to prepare methylthioether and other organic compounds like ethyl bromide. Its hydrolysis in moist air produces methanethiol, which has a low odor threshold and a noxious fecal smell.
Purification Methods
Dissolve the salt (10g) in EtOH (10mL) and add Et2O (100mL). Cool and collect the precipitate, wash it with Et2O and dry it in a vacuum. It is a white powder which is very soluble in EtOH and H2O. [Billmann & Jensen Bull Soc Chim Fr 3 2318 1936, Beilstein 1 III 1212.]
Properties of Sodium thiomethoxide
| Density | 1.12[at 20℃] |
| vapor pressure | 29hPa at 25℃ |
| Flash point: | 27°C |
| storage temp. | Flammables area |
| solubility | soluble in Water |
| form | Powder |
| appearance | White solid |
| color | White to off-white |
| Water Solubility | soluble |
| Merck | 14,5956 |
| BRN | 3592983 |
| InChI | InChI=1S/CH4S.Na/c1-2;/h2H,1H3;/q;+1/p-1 |
| CAS DataBase Reference | 5188-07-8(CAS DataBase Reference) |
| EPA Substance Registry System | Methanethiol, sodium salt (5188-07-8) |
Safety information for Sodium thiomethoxide
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H226:Flammable liquids H302:Acute toxicity,oral H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. |
Computed Descriptors for Sodium thiomethoxide
| InChIKey | RMBAVIFYHOYIFM-UHFFFAOYSA-M |
| SMILES | CS[Na] |
Sodium thiomethoxide manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
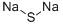
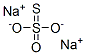
You may like
-
 Methyl Mercaptan Sodium Salt (ca. 15% in Water) CAS 5188-07-8View Details
Methyl Mercaptan Sodium Salt (ca. 15% in Water) CAS 5188-07-8View Details
5188-07-8 -
 Sodium thiomethoxide, 95% CAS 5188-07-8View Details
Sodium thiomethoxide, 95% CAS 5188-07-8View Details
5188-07-8 -
 Sodium thiomethoxide Neat Form CAS 5188-07-8View Details
Sodium thiomethoxide Neat Form CAS 5188-07-8View Details
5188-07-8 -
 Sodium thiomethoxide solution CASView Details
Sodium thiomethoxide solution CASView Details -
 Sodium ThiomethoxideView Details
Sodium ThiomethoxideView Details
5188-07-8 -
 Sodium Methyl Mercaptide apiView Details
Sodium Methyl Mercaptide apiView Details
5188-07-8 -
 SODIUM METHYL MERCAPTANView Details
SODIUM METHYL MERCAPTANView Details
5188-07-8 -
 Sodium ThiomethoxideView Details
Sodium ThiomethoxideView Details
5188-07-8
