Sodium sulfide
Synonym(s):Disodium sulfide
- CAS NO.:1313-82-2
- Empirical Formula: Na2S
- Molecular Weight: 78.04
- MDL number: MFCD00003498
- EINECS: 215-211-5
- Update Date: 2025-12-17 09:49:39
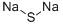
What is Sodium sulfide?
Chemical properties
Sodium sulfide,Na2S, also known as sodium sulfuret,is an irritating, water-soluble, yellowish to reddish, deliquescent powder that melts at 1180°C (2156 °F). Sodium sulfide is used as a chemical intermediate and solvent,in conversion of wood into paper pulp, as a photographic and analytical reagent,as a source of sulfide,as a reducing agent,in organic reactions, as a depilatory, and in sheep dips.
Physical properties
White cubic crystal; hygroscopic; density 1.856 g/cm3; melts at 1,172°C; soluble in water 18.6 g/100mL at 20°C and 39 g/100mL at 50°C; aqueous solutions strongly alkaline; slightly soluble in alcohol; insoluble in ether.
The pentahydrate consists of flat, shiny prismatic crystals; density 1.58 g/cm3; loses three water molecules at 100°C; melts at 120°C losing all water molecules; soluble in water and alcohol; aqueous solutions strongly alkaline; insoluble in ether.
The nonahydrate is a yellowish-white crystalline solid; tetragonal crystals; odor of hydrogen sulfide; the color changes on exposure to light and air, first turning to yellow and then becoming brownish-black, deliquescent; density 1.43 g/cm3; decomposes at about 50°C; very soluble in water; aqueous solution strongly alkaline; slightly soluble in alcohol; insoluble in ether.
The Uses of Sodium sulfide
These yellow flakes were made by fusing sodium carbonate with sulfur. Soluble in water but less so in alcohol, sodium sulfide was lovingly called “stink” by those who used it for toning prints or intensifying negatives because of its sulfurous smell.
The Uses of Sodium sulfide

To a solution of the SM (1 g, 10.3 mmol) in 3:1 EtOH/H2O (26 mL) was added Na2S (1.2 g, 15.4 mmol). The reaction mixture was stirred at RT for 12 h. After completion of the reaction, solvent was removed in vacuo. The resulting residue was diluted with H2O and extracted with EtOAc. The org layer was dried (Na2SO4) and concentrated. The resulting material was purified by silica gel column chromatography to provide the product as a brown solid. [200 mg, 24%]
The Uses of Sodium sulfide
used in sulfur dyes, used as reductant, mordant, floatation agent, depilatory for leather, digestion auxiliary in paper-making, and used in textile, pigment and rubber
The Uses of Sodium sulfide
It is used for H2S therapy, to study its effect on the prevention of diabetes in animals.
The Uses of Sodium sulfide
Sodium sulfide (Na2S) is used in the dye industry, in the oxidation process of gold, lead, and cooper metal ores, as a sheep dip, and to process paper.
Background
Sodium sulfide is a chemical compound with a chemical formula Na2S. Sodium sulfide is used in the pulp and paper industry, water treatment, textile industry, and various chemical manufacturing processes including the production of rubber chemicals, sulfur dyes and oil recovery. Along with its hydrate form, sodium sulfide releases hydrogen sulfide (H2S) when in contact with moist air. H2S is an endogenous gaseous transmitter that exhibits anti-inflammatory and antiapoptotic properties. Along with other sulfides, the anti-inflammatory and tissue-protective effects of sodium sulfide have been investigated in models of inflammation and oxidative stress. Interestingly, sodium sulfide was shown to have some cardioprotective role against cardiac ischemia or reperfusion injury, as well as protect lungs against ventilator-induced lung injury . However the clinical significance and mechanism of action of sodium sulfide and replication of results across other studies are not yet fully determined.
Definition
ChEBI: A sulfide salt with formula Na2S. The pentahydrate and (particularly) the nonahydrate are also known. In gel form, sodium sulfide is used to soften toenails to assist in trimming (and so relive pain) of ingrowing toenails.
Definition
A yellow-red solid, Na2S, formed by the reduction of sodium sulphate with carbon (coke) at elevated temperatures. It is a corrosive and readily oxidized material of variable composition and usually contains polysulphides of the type Na2S2, Na2S3, and Na2S4, which cause the variety of colours. It is known in an anhydrous form (r.d. 1.85; m.p. 1180°C) and as a nonahydrate, Na2S·9H2O (r.d. 1.43; decomposes at 920°C). Other hydrates of sodium sulphide have been reported. The compound is deliquescent, soluble in water with extensive hydrolysis, and slightly soluble in alcohol. It is used in wood pulping, dyestuffs manufacture, and metallurgy on account of its reducing properties. It has also been used for the production of sodium thiosulphate (for the photographic industry) and as a depilatory agent in leather preparation. It is a strong skin irritant.
Preparation
Sodium sulfide is prepared by heating sodium bisulfate with sodium chloride and coal above 950°C. The product mixture is extracted with water and the hydrated sulfide is obtained from the solution by crystallization: NaHSO4 + NaCl + 2C → Na2S + 2CO2↑ + HCl↑
Sodium sulfide also is produced from its elements in liquid ammonia: Na + 2S → Na2S.
General Description
Sodium sulfide is a yellow to brick red crystalline mass or fused solid with an odor of rotten eggs. If exposed to moist air Sodium sulfide is liable to spontaneous heating and may cause ignition of nearby combustible material. Sodium sulfide absorbs moisture from the air.
Air & Water Reactions
Aqueous solutions of sodium sulfide when exposed to air slowly convert to sodium hydroxide and sodium thiosulfate. The crystalline form upon exposure to air forms hydrogen sulfide and sodium carbonate [Merck 11th ed. 1989].
Reactivity Profile
SODIUM SULFIDE is a white to yellow crystalline material, flammable. Can explode on rapid heating or when shocked. Violent reaction with carbon, charcoal, diazonium salts, N,N-dichloromethylamine, strong oxidizers, water. On contact with acids Sodium sulfide liberates highly toxic and flammable hydrogen sulfide gas. When heated to decomposition Sodium sulfide emits toxic fumes of sodium oxide, and oxides of sulfur [Bretherick, 5th ed., 1995, p. 1729].
Hazard
Flammable, dangerous fire and explosion risk. Strong irritant to skin and tissue, liberates toxic hydrogen sulfide on contact with acids.
Health Hazard
Caustic action on skin and eyes. If ingested may liberate hydrogen sulfide in stomach.
Fire Hazard
Special Hazards of Combustion Products: Irritating sulfur dioxide is produced in fire.
Flammability and Explosibility
Non flammable
Industrial uses
In non-metallic flotation, sodium sulfide is also used as a depressant and for collector desorption, in particular, fatty acids from monazite, pyrochlore, zircon and microcline. As a depressant for quartz, sodium sulfide is an excellent depressant for iron-activated quartz as well as non-activated quartz.
Industrial uses
Sodium sulfide (Na2S·9H2O) is a hygroscopic substance with a specific gravity of 1.864 and a melting temperature of 1180 °C. The reagent is soluble in water. The aqueous solution of sodium sulfide has a highly alkaline reaction resulting from its hydrolysis: Na2S + H2O ? NaOH + NaHS
Safety Profile
A poison by ingestion and intraperitoneal routes. Flammable when exposed to heat or flame. Unstable and can explode on rapid heating or percussion. Reacts violently with carbon, diazonium salts, n,n-dichloromethylamine, onitroaniline diazonium salt, water. When heated to decomposition it emits toxic fumes of SOx and Na2O. See also SULFIDES
Metabolism
Not Available
Purification Methods
Some purification of the hydrated salt can be achieved by selecting large crystals and removing the surface layer (contaminated with oxidation products) by washing with distilled water. Other metal ions can be removed from Na2S solutions by passage through a column of Dowex ion-exchange A-1 resin, Na+-form. The hydrated salt can be rendered anhydrous by heating it in a stream of H2 or N2 until water is no longer evolved. (The resulting cake should not be heated to fusion because it is readily oxidised.) Recrystallise it from distilled water [Anderson & Azowlay J Chem Soc, Dalton Trans 469 1986]. Note that sodium sulfide hydrolyses in H2O to form NaHS + H2O, and is therefore alkaline. A 0.1N solution in H2O is 86% hydrolysed at room temperature. Its solubility in H2O is 8% at 0o, 12% at 20o and 30% at 50o. The anhydrous salt is obtained by allowing it to stand in a vacuum over conc H2SO4 or P2O5 at 45o to start with, then at 30-35o when the salt contains 4% of water. The last traces of water are removed by heating to 700o in a glass or porcelain tube in a stream of H2 to give pure H2S. [Fehér in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I pp 358-360 1963.]
Properties of Sodium sulfide
| Melting point: | 950 °C(lit.) |
| Density | 1.86 g/mL at 25 °C(lit.) |
| storage temp. | Refrigerator (+4°C) |
| solubility | H2O: 0.1 g/mL, clear, colorless |
| form | flakes |
| color | Yellow |
| Specific Gravity | 1.856 |
| Water Solubility | 186 g/L (20 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,8681 |
| Dielectric constant | 5.0(Ambient) |
| Stability: | Spontaneously flammable. Incompatible with acids, metals, oxidizing agents. Contact with acid liberates toxic gas. Fine dust/air mixtures are explosive. Hygroscopic. |
| CAS DataBase Reference | 1313-82-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Sodium sulfide(1313-82-2) |
| EPA Substance Registry System | Sodium sulfide (1313-82-2) |
Safety information for Sodium sulfide
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H251:Self-heating substances and mixtures H290:Corrosive to Metals H314:Skin corrosion/irritation H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P235:Keep cool. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Sodium sulfide
| InChIKey | CXPWOVUZRAFMDA-UHFFFAOYSA-N |
Sodium sulfide manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Sodium sulfide 98%View Details
Sodium sulfide 98%View Details -
 Sodium sulfide 99%View Details
Sodium sulfide 99%View Details -
 Sodium Sulphide CAS 1313-82-2View Details
Sodium Sulphide CAS 1313-82-2View Details
1313-82-2 -
 Sodium sulfide, 90%, balance water CAS 1313-82-2View Details
Sodium sulfide, 90%, balance water CAS 1313-82-2View Details
1313-82-2 -
 Sodium Sulfide SNa2 50% CAS 1313-82-2View Details
Sodium Sulfide SNa2 50% CAS 1313-82-2View Details
1313-82-2 -
 Sodium sulfide 60% CAS 1313-82-2View Details
Sodium sulfide 60% CAS 1313-82-2View Details
1313-82-2 -
 Sodium Sulphide - Yellow FlakesView Details
Sodium Sulphide - Yellow FlakesView Details
1313-82-2 -
 Sodium SulphideView Details
Sodium SulphideView Details
1313-82-2
