Sodium thiosulfate
Synonym(s):Sodium thiosulfate;Sodium thiosulphate;Sodium thiosulfate pentahydrate;Sodium hyposulfite;Hypo
- CAS NO.:7772-98-7
- Empirical Formula: Na2O3S2
- Molecular Weight: 158.11
- MDL number: MFCD00003499
- EINECS: 231-867-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:44
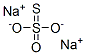
What is Sodium thiosulfate?
Description
Sodium thiosulfate is a common inorganic compound that is stable, environmentally friendly and inexpensive. It can be used in synthetic chemistry to facilitate the synthesis of a series of diamine-copper chlorinated naphthalene complexes through a biochemical process assisted by sodium thiosulfate. The complexes have potential applications in catalysis, materials science and as precursors for the synthesis of more complex molecular structures.
Background
Sodium thiosulfate (thiosulfate) Known simply as “hypo,” an abbreviated form of the incorrect form hyposulfite, this white crystal was made by boiling calcium thiosulfate with sodium sulfate. It is soluble in water and oil of turpentine but not in alcohol. The property of sodium thiosulfate to dissolve silver halides was discovered in 1819 by Sir John Herschel and was probably first used to fix photographic images by L. J. M. Daguerre. After Daguerre published its use in his 1839 manual W. H. F. Talbot finally used it to fix calotype negatives and silver chloride prints, although he continued to stabilize them with potassium iodide and sodium chloride presumably for the variety of colors possible. Sodium thiosulfate was the primary fixing agent throughout the 19th century and is still used today.
Chemical properties
Sodium thiosulfate, Na2S203·5H20, also known as sodium hyposulfite, hypo,andsodium subsulfite, is a white crystalline solid that has a melting point of 48 °C(118 °F). Water soluble,it is used as a fixing agent for photographic films, plates, and papers. Sodium thiosulfate is used in medicine, as a germicide, in manufacturing leather, as a mordant in dyeing, and for extracting silver from ore.
Physical properties
Anhydrous thiosulfate is a white powder; soluble in water; insoluble in ethanol.
Sodium pentahydrate is a colorless, odorless, crystalline solid; density 1.69 g/cm3; decomposes around 50°C; effloresces in dry air above 33°C; very soluble in water and oil of turpentine; insoluble in ethanol.
The Uses of Sodium thiosulfate
Sodium thiosulfate is a common analytical reagent used in iodometric titration to analyze chlorine, bromine, and sulfide. Other uses are in bleaching paper pulp, bleaching straw, ivory, and bones, for removing chlorine from solutions, silver extraction from its ores, a mordant in dyeing and printing textiles, and as an antidote to cyanide poisoning.
Another major application is in photography, where it is used as a fixer to dissolve unchanged silver salts from exposed negatives. It is mainly used as a prophylactic against cyanide poisoning in the pharmaceutical field, where it converts cyanide into thiocyanate, a reaction catalysed by the enzyme Rhodanese. In addition, it is a chelating agent, antioxidant and formulation aid, and is a water-soluble powder that is used in alcoholic beverages at a dosage of 5 ppm and in table salt at a dosage of 0.1 per cent.
What are the applications of Application
Sodium thiosulfate is a widely useful thiosulfate-assisted biochemical used for synthesis of a variety of diamine-CuCN complexes
Definition
ChEBI: Sodium thiosulfate is an inorganic sodium salt composed of sodium and thiosulfate ions in a 2:1 ratio. It has a role as an antidote to cyanide poisoning, a nephroprotective agent and an antifungal drug. It contains a thiosulfate(2-).
brand name
Sulfactol (Sterling Winthrop).
General Description
Sodium thiosulfate (Na2S2O3) can be obtained from Na2S2O3.10H2O by heating above 100 °C. It can be synthesized from sodium sulfate.
Reaction
Sodium thiosulfate is a common reducing agent. It reduces iodine to iodide anion forming sodium tetrathionate. This reaction is utilized in the so-called iodometric titration: 2S2O32ˉ + I2 → S4O62ˉ + 2Iˉ.
It reacts with chlorine to form sodium bisulfate and hydrochloric acid. This reaction removes chlorine from aqueous solutions: Na2S2O3 + 4Cl2 + 5H2O → 2NaHSO4 + 8HCl.
It also reacts with hydrochloric acid, decomposing to sulfur and sulfur dioxide: Na2S2O3 + 2HCl → 2NaCl + S + SO2 + H2O.
Hazard
Use in foods restricted to 0.1%.
Safety Profile
Moderately toxic by subcutaneous route. Incompatible with metal nitrates, sodium nitrite. When heated to decomposition it emits very toxic fumes of Na2O and SOx. See also SODIUM THIOSULFATE and SODIUM THIOSULFATE, PENTAHYDRATE.
Veterinary Drugs and Treatments
Sodium thiosulfate (alone or in combination with sodium nitrite)
is useful in the treatment of cyanide toxicity. It has been touted for
use in treating arsenic or other heavy metal poisonings, but its efficacy
is in question for these purposes. However, because sodium
thiosulfate is relatively non-toxic and inexpensive, it may be tried
to treat arsenic poisoning. When used in combination with sodium
molybdate, sodium thiosulfate may be useful for the treatment of
copper poisoning.
Sodium thiosulfate may be useful for the topical treatment for
some fungal infections (Tinea). In humans, sodium thiosulfate has
been used to reduce the nephrotoxicity of cisplatin therapy. A 3 or
4% solution has been used to infiltrate the site of extravasations of
cisplatin, carboplatin, or dactinomycin. In combination with steroids,
sodium thiosulfate may reduce the healing time associated
with doxorubicin extravasation.
Purification Methods
Crystallise it from EtOH/H2O solutions or from water (0.3mL/g) below 60o by cooling to 0o, and dry it at 35o over P2O5 under vacuum. [Foerster & Mommsen Chem Ber 57 258 1924.] This salt is used as a secondary standard in volumetric analysis [Kilpatrick J Am Chem Soc 45 2132 1923], and is used as “Hypo” in photography [Hargreaves & Dunningham J Soc Chem Ind 42 147T 1923.]
Properties of Sodium thiosulfate
| Melting point: | 48°C |
| Boiling point: | 100°C |
| Density | 1.01 g/mL at 25 °C |
| storage temp. | Store at +5°C to +30°C. |
| solubility | Immiscible with alcohol. |
| form | Solid |
| Specific Gravity | 1.667 |
| color | White |
| PH | 6.0-8.5 (25℃, 50mg/mL in H2O) |
| Water Solubility | Soluble in water. Insoluble in alcohol. |
| Sensitive | Hygroscopic |
| Merck | 14,8694 |
| Stability: | Stable. Incompatible with strong acids, strong oxidizing agents, iodine, mercury. |
| CAS DataBase Reference | 7772-98-7(CAS DataBase Reference) |
| EPA Substance Registry System | Sodium thiosulfate (7772-98-7) |
Safety information for Sodium thiosulfate
Computed Descriptors for Sodium thiosulfate
| InChIKey | AKHNMLFCWUSKQB-UHFFFAOYSA-L |
Sodium thiosulfate manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Sodium Thio Sulphate 98%View Details
Sodium Thio Sulphate 98%View Details -
 SODIUM THIO SULPHATE 99%View Details
SODIUM THIO SULPHATE 99%View Details -
 Sodium Thio Sulphate 99%View Details
Sodium Thio Sulphate 99%View Details -
 SODIUM THIO SULPHATE 98%View Details
SODIUM THIO SULPHATE 98%View Details -
 Sodium thiosulfate 99%View Details
Sodium thiosulfate 99%View Details -
 Sodium thiosulfate 98%View Details
Sodium thiosulfate 98%View Details -
 10102-17-71 SODIUM THIOSULPHATE 0.05N SOLUTION 99%View Details
10102-17-71 SODIUM THIOSULPHATE 0.05N SOLUTION 99%View Details
10102-17-71 -
 Sodium Thio SulphateView Details
Sodium Thio SulphateView Details
10102-17-7
