Sodium Phosphate, Dibasic
Synonym(s):Disodium phosphate;Sodium phosphate dibasic;Disodium hydrogen phosphate;Na?HPO?;Sodium hydrogenphosphate
- CAS NO.:7558-79-4
- Empirical Formula: Na2HPO4
- Molecular Weight: 141.96
- MDL number: MFCD00003496
- EINECS: 231-448-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:22
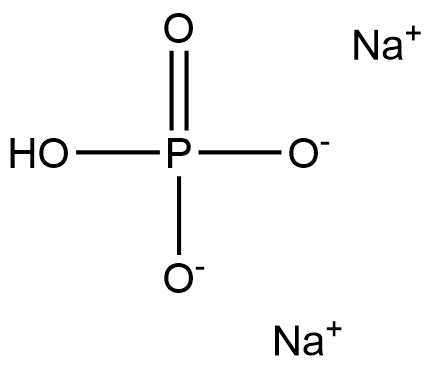
What is Sodium Phosphate, Dibasic?
Absorption
Tmax for phosphate absorption with orally administered liquid sodium phosphate is 1-3h .
Toxicity
Intamuscular LD50 of 250mg/kg and oral LD50 of 8290 mg/kg reported in rats . Phosphate toxicity is likely due to the disturbance of other electrolytes when phosphate levels are high, producing symptoms including tetany, dehydration, hypotension, tachycardia, hyperpyrexia, cardiac arrest and coma . Risk of raising phosphate levels through use of sodium phosphate appears to be higher in smaller patients .
Description
Sodium phosphate dibasic is highly hygroscopic and water soluble. Useful in conjunction with Sodium Phosphate, Monobasic in the preparation of biological buffers. It is commonly used in biological assay buffers. Sodium is required for nerve and muscle functioning. Sodium Phosphate Dibasic can be used as a dietary supplement and as a nutrient. Sodium hydrogen phosphate is used in food and water treatment in conjunction with trisodium phosphate.In foods, it is used to adjust pH. In water treatment, It retards calcium scale formation. It is also found in some detergents and cleaning agents. This product is known to be Used in many applications including the purification of antibodies. It is used as a saline laxative to treat constipation or to clean the bowel before a colonoscopy.
Chemical properties
Sodium hydrogen phosphate,NaH2P04·H20, is hygroscopic, transparent, water-soluble crystals. Used as a purgative, reagent,and buffer.
Chemical properties
The anhydrous compound is white crystalline powder; hygroscopic; density 1.70 g/cm3; converts to sodium pyrophosphate at 240°C; soluble in water; 874 SODIUM PHOSPHATE, DIBASIC insoluble in alcohol. The dihydrate is a crystalline powder or granular solid; density 2.066 g/cm3 at 15°C; loses water of crystallization at 92.5°C. The heptahydrate is a powdered or granular crystalline solid; density 1.679 g/cm3; loses five molecules of water at 48°C. The dodecahydrate is made up of translucent crystals or granules; density 1.524 g/cm3; readily loses five water molecules on exposure to air at ambient temperature; melts at 35°C when the salt contains all water of crystallization; loses all water at 100°C. All the hydrates are soluble in water and insoluble in alcohol.
Chemical properties
Disodium phosphate is anhydrous or contains two molecules of water of hydration
Physical properties
The anhydrous compound is white crystalline powder; hygroscopic; density 1.70 g/cm3; converts to sodium pyrophosphate at 240°C; soluble in water;insoluble in alcohol.
The dihydrate is a crystalline powder or granular solid; density 2.066 g/cm3 at 15°C; loses water of crystallization at 92.5°C.
The heptahydrate is a powdered or granular crystalline solid; density 1.679 g/cm3; loses five molecules of water at 48°C.
The dodecahydrate is made up of translucent crystals or granules; density 1.524 g/cm3; readily loses five water molecules on exposure to air at ambient temperature; melts at 35°C when the salt contains all water of crystallization; loses all water at 100°C.
All the hydrates are soluble in water and insoluble in alcohol.
Occurrence
Disodium phosphate is anhydrous or contains two molecules of water of hydration
The Uses of Sodium Phosphate, Dibasic
Sodium Phosphate, Dibasic is used as a laboratory reagent and a buffer in chemical analysis. Other uses are in manufacturing ceramics, detergents, and enamels; as a mordant in dyeing; for fireproofing paper and wood; for weighting and printing silk; in the treatment of boiler water; as a sequestrant in food; as a dietary supplement; in soldering enamels; and in fertilizers. It is used therapeutically as a cathartic.
The Uses of Sodium Phosphate, Dibasic
It is used in pH buffer solutions.
The Uses of Sodium Phosphate, Dibasic
Sodium Phosphate Dibasic is used as an in conjunction with trisodium phosphate in foods and water treatment. In foods, it is used to adjust pH. In water treatment, It retards calcium scale formation. It is also found in some detergents and cleaning agents. Sodium Phosphate Dibasic is used as a saline laxative to treat constipation or to clean the bowel before a colonoscopy.
Indications
Used to treat constipation or to clean the bowel before a colonoscopy .
What are the applications of Application
Sodium phosphate dibasic solution is for the preparation of phosphate buffers used in biological applications
What are the applications of Application
Sodium Phosphate, Dibasic is reagent with high buffering capacity for molecular biology, biochemistry, and chromatography.
Preparation
Dibasic sodium phosphate is prepared by treating phosphoric acid with a slight excess of sodium carbonate. The solution is boiled to expel carbon dioxide. Upon cooling dodecahydrate crystallizes out. Heating dodecahydrate at 100°C forms the anhydrous salt.
H3PO4 + Na2CO3 → Na2HPO4 + CO2 + H2O
Dibasic sodium phosphate also is prepared by reacting dibasic calcium phosphate with sodium carbonate. The product calcium carbonate precipitates leaving dibasic sodium salt in the solution. The solution on cooling yields crystals of hydrated product.
CaHPO4 + Na2CO3 → CaCO3 + Na2HPO4
Definition
ChEBI: Disodium hydrogenphosphate is a sodium phosphate.
General Description
Sodium phosphate dibasic in combination with sodium phosphate monobasic is used in the preparation of oral sodium phosphate solution (OSPS), which is a purgative used for bowel cleansing before colonoscopy.
Flammability and Explosibility
Non flammable
Biochem/physiol Actions
Sodium phosphate dibasic is an important component of running buffer of denaturing gel electrophoresis. It induces glomerular calcification as well as other nephrotic syndrome in Sprague-Dawley rats.
Pharmacokinetics
Sodium phosphate inceases fecal water content to increase mobility through the large intestine .
Safety Profile
Poison by intravenous route. Moderately toxic by intraperitoneal, subcutaneous, and intramuscular routes. Mildly toxic by ingestion. A skin and eye irritant. When heated to decomposition it emits toxic fumes of POx and Na2O. See also PHOSPHATES.
Metabolism
Not Available
Purification Methods
Crystallise the salt twice from warm water, by cooling. Dry in air, then in an oven overnight at 130o. It should be dried before use as it is slightly hygroscopic. It forms di-, hepta-and deca-hydrates.
Properties of Sodium Phosphate, Dibasic
| Melting point: | 243-245 °C |
| Density | 1.064 g/mL at 20 °C |
| vapor density | 4.9 (vs air) |
| vapor pressure | 0Pa at 20℃ |
| FEMA | 2398 | DISODIUM PHOSPHATE |
| storage temp. | Store at +5°C to +30°C. |
| solubility | H2O: 0.5 M at 20 °C, clear, colorless |
| form | powder |
| appearance | White crystalline solid |
| color | White |
| PH | 8.9-9.2 (25℃, 50g/L in water) |
| pka | (1) 2.15, (2) 6.82, (3) 12.38 (at 25℃) |
| Odor | odorless |
| Water Solubility | >=10 g/100 mL at 20 ºC |
| λmax | λ: 260 nm Amax: 0.019 λ: 280 nm Amax: 0.016 |
| Sensitive | Hygroscopic |
| Merck | 14,8659 |
| Stability: | Stable. Incompatible with strong acids. Hygroscopic. |
| CAS DataBase Reference | 7558-79-4(CAS DataBase Reference) |
| EPA Substance Registry System | Disodium phosphate (7558-79-4) |
Safety information for Sodium Phosphate, Dibasic
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Sodium Phosphate, Dibasic
| InChIKey | BNIILDVGGAEEIG-UHFFFAOYSA-L |
Sodium Phosphate, Dibasic manufacturer
JSK Chemicals
Arcube Science
Pachisia Chemical Works
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Sodium hydrogen phosphate, anhydrous CAS 7558-79-4View Details
Sodium hydrogen phosphate, anhydrous CAS 7558-79-4View Details
7558-79-4 -
 Sodium hydrogen phosphate, anhydrous CAS 7558-79-4View Details
Sodium hydrogen phosphate, anhydrous CAS 7558-79-4View Details
7558-79-4 -
 Sodium Phosphate Dibasic Anhydrous for cell culture CAS 7558-79-4View Details
Sodium Phosphate Dibasic Anhydrous for cell culture CAS 7558-79-4View Details
7558-79-4 -
 Sodium Phosphate Dibasic Anhydrous extrapure CAS 7558-79-4View Details
Sodium Phosphate Dibasic Anhydrous extrapure CAS 7558-79-4View Details
7558-79-4 -
 Sodium Phosphate Dibasic Anhydrous ACS CAS 7558-79-4View Details
Sodium Phosphate Dibasic Anhydrous ACS CAS 7558-79-4View Details
7558-79-4 -
 Solid Di Sodium Phosphate, Grade Standard: Industrial GradeView Details
Solid Di Sodium Phosphate, Grade Standard: Industrial GradeView Details
7558-79-4 -
 Liquid Disodium Phosphate, Grade Standard: Reagent GradeView Details
Liquid Disodium Phosphate, Grade Standard: Reagent GradeView Details
7558-79-4 -
 8.7-9.3 Powder Di - Sodium Phosphate Dihydrate (Pure Grade), For IndustrialView Details
8.7-9.3 Powder Di - Sodium Phosphate Dihydrate (Pure Grade), For IndustrialView Details
10028-24-7
