Ammonium oxalate
- CAS NO.:1113-38-8
- Empirical Formula: C2H2O4.2H3N
- Molecular Weight: 124.1
- MDL number: MFCD00013308
- EINECS: 214-202-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
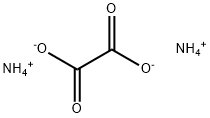
What is Ammonium oxalate?
Description
Ammonium oxalate, C2H8N2O4 (some times written as (NH4)2C2O4), is an oxalate salt with ammonium (sometimes as a monohydrate). It is a constituent of some types of kidney stone. Found also in guano.
Chemical properties
Ammonium oxalate is an odorless, colorless crystalline material or powder.
The Uses of Ammonium oxalate
Ammonium oxalate is used as an analytical reagent and general reducing agent. It is commonly employed in soil chemical analysis to extract iron and aluminum from poorly-crystal line minerals.
Definition
ChEBI: An ammonium salt consisting of ammonium and oxalate ions in a 2:1 ratio.
Flammability and Explosibility
Not classified
Safety Profile
A poison. Can react violently with (NaOCl+ ammonium acetate). When heated to decomposition it SYNS: ETHANEDIOIC ACID DIAMMONIUN SALT 0 OXALIC ACID, DIAIMMONIUM SALT can emit toxic fumes of NH3 and NOx. See also OXALATES.
Potential Exposure
It is used in chemical analysis and to make blueprint paper, explosives; a rust-removal ingredient in metal polishes.
Shipping
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required. UN1759 Corrosive solids, n.o.s., Hazard class: 8; Labels: 8-Corrosive material, Technical Name Required.
Incompatibilities
Ammonium oxalate is a reducing agent and also reacts as a base to neutralize acids and reacts with oxidizers generating carbon dioxide. Incompatible with oxidizers (chlorates, hypochlorite solutions, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.
Properties of Ammonium oxalate
| Melting point: | 222-224°C |
| Boiling point: | 230.85°C (rough estimate) |
| Density | 1.5000 |
| vapor pressure | 0Pa at 20℃ |
| refractive index | 1.4350 (estimate) |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | Water (Slightly, Sonicated) |
| form | Solid |
| color | Colorless crystals |
| Odor | odorless |
| Water Solubility | Soluble |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 1113-38-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Ammonium oxalate(1113-38-8) |
| EPA Substance Registry System | Diammonium oxalate (1113-38-8) |
Safety information for Ammonium oxalate
Computed Descriptors for Ammonium oxalate
Ammonium oxalate manufacturer
ARRAKIS INDUSTRIES LLP
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran


You may like
-
 1113-38-8 Ammonium Oxalate 99%View Details
1113-38-8 Ammonium Oxalate 99%View Details
1113-38-8 -
 Ammonium Oxalate 99%View Details
Ammonium Oxalate 99%View Details -
 Ammonium oxalate 98%View Details
Ammonium oxalate 98%View Details -
 Ammonium oxalate 1113-38-8 98%View Details
Ammonium oxalate 1113-38-8 98%View Details
1113-38-8 -
 1113-38-8 Ammonium oxalate 99%View Details
1113-38-8 Ammonium oxalate 99%View Details
1113-38-8 -
 1113-38-8 99%View Details
1113-38-8 99%View Details
1113-38-8 -
 AMMONIUM OXALATE 1113-38-8 99%View Details
AMMONIUM OXALATE 1113-38-8 99%View Details
1113-38-8 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
