Calcium silicate
Synonym(s):Calcium metasilicate;Calcium silicon oxide;Silicon calcium oxide
- CAS NO.:1344-95-2
- Empirical Formula: CaO3Si
- Molecular Weight: 116.16
- MDL number: MFCD00015979
- EINECS: 935-756-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:37
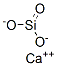
What is Calcium silicate?
Description
Calcium Silicate is a hydrous or anhydrous silicate with varying proportions of calcium oxideand silica. many different calcium silicates occur in nature in mineral form (e.g.,wollastonite, afwillite, grammite). The stoichiometry of the hydrate in cement paste is variable and the state of the chemically and physically bound water in its structure is not clear. Thus, calcium silicate can be prepared by calcining the oxide with silica, SiO2, at an elevated temperature: CaO + SiO2+ heat→CaSiO3. Calcium orthosilicate is commonly used as a safe alternative to asbestos for high-temperature insulation materials. Industrial grade piping and equipment insulation are often fabricated from calcium orthosilicate.
Chemical properties
Calcium silicate occurs as a crystalline or amorphous white or off white material, and often exists in different hydrate forms. It consists of different ratios of CaO and SiO4, including tricalcium silicate (3CaO.SiO2) and dicalcium silicate Ca2SiO4. It is insoluble in water and forms a gel with inorganic acids. The pH of 5% suspension is 8.4~10.2.
The Uses of Calcium silicate
Calcium Silicate is an anticaking agent that exists in different forms, which are insoluble in water. it is used in salt to enhance flowability under extremely high humidity conditions. it is also used in baking powder and fabricated chips to absorb water or other liquids.
Production Methods
Calcium silicate is made in various ways by reacting siliceous material (such as diatomaceous earth) and calcium compounds (such as calcium hydroxide (Ca (OH) 2).The refinement of wollastonite ore into high-grade wollastonite was originally done by manual selection at many mines. This process is now performed by screening and magnetic separators, sometimes in combination with flotation and vacuum filtration. Grinding and milling operations can produce variable mesh powders or aggregates.
What are the applications of Application
The main applications of calcium silicate relate to its anticaking properties, and it has therefore been used in dusting powders and a range of different cosmetic products (e.g. face powders, eye shadow). It is also used as anticaking agent in table salt, foods, pharmaceuticals, and agricultural pesticides; replacement for asbestos in thermal insulation; Constituent (produced in situ) of lime glass, portland cement; reinforcing filler in elastomers and plastics; absorbent for liquids, gases, vapors; as anti-caking agent, suspension agent, pigment and pigment extender; binder for refractory material; in chromatography; in road construction.
Preparation
Calcium silicate is a naturally occurring mineral, but for commercial applications it is usually prepared from lime and diatomaceous earth under carefully controlled conditions.
Hazard
Irritating dust. Use in foods restricted to 5% in baking powder, 2% in table salt. Upper res- piratory tract irritant. Questionable carcinogen.
Health Hazard
The toxicity of calcium silicate
depends on particle size, aspect ratio, and
amount of silica and respirable fiber.1 Synthetic
nonfibrous calcium silicate is considered to be
a nuisance dust.
Skin irritation was reported in a worker
exposed to an atmosphere permeated with calcium silicate.1 A study of 104 wallastonite (a
naturally occurring calcium silicate mineral)
miners showed no relationship between the
prevalence of chronic bronchitis or airflow
obstruction with increasing exposure.1 In a
cohort mortality study of wollastonite quarry
workers the observed numbers of deaths from
all cancers combined and lung cancer were
lower than expected.
Flammability and Explosibility
Not classified
Pharmaceutical Applications
Calcium silicate (Ca2SiO4) is used in the pharmaceutical industry as an anticaking agent. Anti-caking agents absorb moisture and allow products to flow freely during the manufacturing process. It is also used as a filler aid for oral pharmaceuticals. It has also been used in pharmaceutical preparations as an antacid.
Industrial uses
Calcium silicate compounds belong to a complicated class of silicates. Among their many industrial applications, calcium silicates are heavily used as a building material as they constitute the main ingredient in today’s cement clinker.
Safety Profile
A nuisance dust
Safety
GRAS listed. Included in the FDA Inactive Ingredients Database (oral dosage forms). Included in nonparenteral (oral, orodispersible, effervescent and enteric-coated tablets) formulations licensed in the UK.
calcium silicate is generally recognized as safe (GRAS) when used at levels not exceed 2% in table salt and 5% in baking powder.
Storage
Calcium silicate is chemically stable and nonflammable, but it should be protected from moisture. Store in airtight containers in a cool, dry place.
Incompatibilities
None reported [Note: After prolonged contact with water, solution reverts to soluble calcium salts & amorphous silica.]
Regulatory Status
Calcium silicate (E552) is listed in Commission Regulation (EU) No 231/2012 as an authorised food additive and categorized as “additives other than colours and sweeteners”. It is an approved ingredient in Australia and New Zealand with the code number 552.
Properties of Calcium silicate
| Melting point: | 1540℃ [CRC10] |
| Density | 2.9 g/mL at 25 °C (lit.) |
| solubility | Practically insoluble in water; forms a gel with mineral
acids. It can absorb up to 2.5 times its weight of liquids and still
remain a free-flowing powder. |
| form | white monoclinic crystals |
| Specific Gravity | 2.9 |
| color | white monoclinic crystals, crystalline |
| Odor | at 100.00?%. odorless |
| PH | 9.5-11.5 (5% in H2O) |
| Water Solubility | insoluble H2O [HAW93] |
| Merck | 13,1707 |
| Stability: | Stable. |
| CAS DataBase Reference | 1344-95-2(CAS DataBase Reference) |
| EPA Substance Registry System | Calcium silicate (1344-95-2) |
Safety information for Calcium silicate
Computed Descriptors for Calcium silicate
Calcium silicate manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


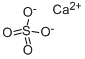
You may like
-
 CALCIUM SILICATE 99%View Details
CALCIUM SILICATE 99%View Details -
 Calcium silicate 98%View Details
Calcium silicate 98%View Details
1344-95-2 -
 Calcium silicate 1344-95-2 98%View Details
Calcium silicate 1344-95-2 98%View Details
1344-95-2 -
 Calcium silicate CAS 1344-95-2View Details
Calcium silicate CAS 1344-95-2View Details
1344-95-2 -
 Calcium silicate CAS 1344-95-2View Details
Calcium silicate CAS 1344-95-2View Details
1344-95-2 -
 Powder Calcium Silicate CAS: 1344-95-2View Details
Powder Calcium Silicate CAS: 1344-95-2View Details
1344-95-2 -
 Calcium Silicate USP/USP NF/FCC, Bag, 25 kgView Details
Calcium Silicate USP/USP NF/FCC, Bag, 25 kgView Details
1344-95-2 -
 Powder Calcium Silicate, For IndustrialView Details
Powder Calcium Silicate, For IndustrialView Details
1344-95-2
