Barium fluoride
Synonym(s):Barium difluoride;Barium fluoride
- CAS NO.:7787-32-8
- Empirical Formula: BaF2
- Molecular Weight: 175.32
- MDL number: MFCD00003450
- EINECS: 232-108-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
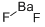
What is Barium fluoride?
Description
Barium fluoride appears as colourless, odourless crystals/white powder and is slightly soluble in water but soluble in ethanol and methanol. When heated, barium fluoride decomposes and emits toxic fumes of fluorine and barium. Barium fluoride is used as a preopacifying agent and in enamel and glazing frits production. Its other use is in the production of welding agents (an additive to some fluxes, a component of coatings for welding rods and in welding powders). It is also used in metallurgy as a molten bath for refining aluminium.
Chemical properties
Barium fluoride, BaF2, is a white crystal or powder.Under the microscope crystals may be clear and colorless. Barium fluoride is used commercially in combination with other fluorides for arc welding (qv) electrode fluxes.
Physical properties
Barium fluoride (BaF2), also known as barium(II) fluoride, is a solid which can also be a transparent crystal. Its molecular weight is 175.34 g/mol. It occurs in nature as the mineral “Frankdicksonite”. It occurs as a white powder with a melting point of 1290 and a boiling point of 2259°C. It is slightly soluble in water (0.159 at 0.0°C and 0.162 g/100 ml of water at 20°C). The solid generally assumes the fluorite structure, but when subjected to high pressure, changes to the PbCl2 structure. In the vapor phase, the BaF2 molecule is nonlinear with an F–Ba–F angle of approximately 108°C.
The Uses of Barium fluoride
Barium Fluoride is used in ceramic flux, carbon brushes for electrical equipment, glass making, in the manufacture of other fluorides, crystals for spectroscopy, electronics and dry-film lubricants. Barium Fluoride is used in spectroscopic components. Barium Fluoride is often suitable for applications in the passive IR band (8 to 14 ?m) and is often used as a viewport window for thermography.
The Uses of Barium fluoride
As a flux and opacifier in vitreous enamels; in the manufacture of carbon brushes for DC motors and generators; in heat-treating metals; in embalming; in glass manufacture.
Production Methods
A single crystal of Barium fluoride is grown from the melt solution by the Kyropoulos method and the Stockbarger method.
What are the applications of Application
Barium fluoride is used as the transmission window in λ: 150 nm–10 mm and as the Reststrahlen filter in λ: 30–60 mm.
Flammability and Explosibility
Non flammable
Safety Profile
A poison by ingestion and intraperitoneal routes. Moderately toxic by subcutaneous route. An experimental teratogen. See also FLUORIDES and BARIUM COMPOUNDS (soluble). When heated to decomposition it emits toxic fumes of F-.
Solubility in water
Barium fluoride is soluble in water with the solubility of 0.17 g/100 g H2O (10 ℃). It is also soluble in acid and ammonium chloride.
Purification Methods
Wash it well with distilled H2O and dry it in a vacuum. Its solubility in H2O is 1.6g (10o), 1.6g (20o) and 1.62g (30o) per L, and is soluble in mineral acids and aqueous NH4Cl. It may be stored in glass bottles. [Kwasnik in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 234 1963.]
Structure and conformation
The space lattice of Barium fluoride (BaF2) belongs to the cubic system, and its calcium fluoride structure has a lattice constant of a=0.6187 nm and Ba–F=0.268 nm.
Properties of Barium fluoride
| Melting point: | 1354 °C |
| Boiling point: | 2260 °C(lit.) |
| Density | 4.89 g/mL at 25 °C(lit.) |
| vapor pressure | 0Pa at 20℃ |
| refractive index | 1.4741 |
| Flash point: | 2260°C |
| storage temp. | Store at +5°C to +30°C. |
| solubility | 1.2g/l |
| form | random crystals |
| Specific Gravity | 4.89 |
| color | White |
| Water Solubility | 1.6 g/L (20 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,974 |
| Solubility Product Constant (Ksp) | pKsp: 6.74 |
| Dielectric constant | 7.3600000000000003 |
| Exposure limits | ACGIH: TWA 2.5 mg/m3 NIOSH: IDLH 250 mg/m3; TWA 2.5 mg/m3 |
| Stability: | Stable. Incompatible with acids. |
| CAS DataBase Reference | 7787-32-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Barium fluoride(7787-32-8) |
| EPA Substance Registry System | Barium fluoride (BaF2) (7787-32-8) |
Safety information for Barium fluoride
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H332:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Barium fluoride
| InChIKey | OYLGJCQECKOTOL-UHFFFAOYSA-L |
Barium fluoride manufacturer
JSK Chemicals
Madras Fluorine Private Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Barium fluoride 98%View Details
Barium fluoride 98%View Details -
 Barium fluoride crystal optic disc, 13mm x 1mm, Polished both sides CASView Details
Barium fluoride crystal optic disc, 13mm x 1mm, Polished both sides CASView Details -
 Barium fluoride crystal optic disc, 25mm x 2mm, Polished both sides CASView Details
Barium fluoride crystal optic disc, 25mm x 2mm, Polished both sides CASView Details -
 Barium fluoride, Ultra dry CAS 7787-32-8View Details
Barium fluoride, Ultra dry CAS 7787-32-8View Details
7787-32-8 -
 Barium fluoride, Ultra dry CAS 7787-32-8View Details
Barium fluoride, Ultra dry CAS 7787-32-8View Details
7787-32-8 -
 Barium fluoride CAS 7787-32-8View Details
Barium fluoride CAS 7787-32-8View Details
7787-32-8 -
 Barium fluoride CAS 7787-32-8View Details
Barium fluoride CAS 7787-32-8View Details
7787-32-8 -
 Barium fluoride CAS 7787-32-8View Details
Barium fluoride CAS 7787-32-8View Details
7787-32-8