Rubidium carbonate
Synonym(s):Carbonic acid dirubidium;Dirubidium carboxide;Rubidium carbonate
- CAS NO.:584-09-8
- Empirical Formula: CH3O3Rb
- Molecular Weight: 148.5
- MDL number: MFCD00011188
- EINECS: 209-530-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
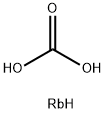
What is Rubidium carbonate?
Description
Rubidium carbonate is a white powder or colorless crystal with a monoclinic crystal structure. Its chemical formula is Rb2CO3. Rubidium is often sold as rubidium carbonate The chemical properties of rubidium carbonate are stable. Rubidium carbonate is very hygroscopic, soluble in water, and insoluble in alcohol. It can be converted to other rubidium compounds by heating or by reacting with acids. Rubidium carbonate is used in the preparation of rubidium metal, other rubidium salts,and rubidium single crystals. It is also used as a specific glass additives, catalysts, analytical reagents and others. For example, rubidium carbonate can be incorporated into the metal oxide electron transport layers, which can improve the lifetime of quantum-dot light emitting diodes[1]. It can also exist as an alkaline in the high-temperature nucleophile synthesis of a semi-crystalline, aromatic poly(ether ketone)[2].
Chemical properties
White powder.Extremely hygroscopic; soluble in water. Dissociates above 900C. The thermal decomposition of rubidium carbonate to produce rubidium oxide and carbon dioxide. This reaction takes place at a temperature of over 900°C in vacuo. The decomposition of Rb2CO3·3H2O2 and Cs2CO3·3H2O2 under isothermal conditions was studied (55–85°) and it was shown that the decomposition of these compounds is self-accelerated. The final products are M2CO3, H2O, and O2. Rb2CO3·3H2O2 has a much higher thermal stability than Cs2CO3·3H2O2.
The Uses of Rubidium carbonate
Rubidium carbonate (Rb2O3) is used to make special types of glass.
The Uses of Rubidium carbonate
Special glass formulations.
The Uses of Rubidium carbonate
Rubidium carbonate is used as a raw materials for preparation of rubidium metal and various rubidium salts, for the manufacturing of special glass, for the manufacturing of high energy density micro cells and crystal scintillation counters. It is also used as a part of a catalyst for preparing short-chain alcohols from feed gas.
Hazard
Strong irritant to tissue.
References
[1] Yujin Lee, Hyo-Min Kim, Jeonggi Kim, Jin Jang, Remarkable lifetime improvement of quantum-dot light emitting diodes by incorporating rubidium carbonate in metal-oxide electron transport layers, Journal of Materials Chemistry C, 2019, 7, 10082-10091.
[2] Kate JC Lim, Paul Cross, Peter Mills, Howard M Colquhoun, Controlled variation of monomer sequence distribution in the synthesis of aromatic poly(ether ketone)s, 2016, 28.
Properties of Rubidium carbonate
| Melting point: | 837 °C |
| Boiling point: | decomposes at 740℃ [KIR82] |
| storage temp. | Store below +30°C. |
| solubility | 4500g/l |
| form | Powder |
| color | White |
| PH | 11.7 (50g/l, H2O, 20℃) |
| Water Solubility | soluble |
| Sensitive | Hygroscopic |
| InChI | InChI=1S/CH2O3.Rb.H/c2-1(3)4;;/h(H2,2,3,4);; |
| CAS DataBase Reference | 584-09-8(CAS DataBase Reference) |
| EPA Substance Registry System | Carbonic acid, dirubidium salt (584-09-8) |
Safety information for Rubidium carbonate
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Rubidium carbonate
| InChIKey | WPFGFHJALYCVMO-UHFFFAOYSA-L |
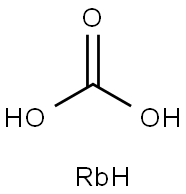
| SMILES | C(O)(O)=O.[RbH] |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Rubidium carbonate CAS 584-09-8View Details
Rubidium carbonate CAS 584-09-8View Details
584-09-8 -
 Rubidium carbonate CAS 584-09-8View Details
Rubidium carbonate CAS 584-09-8View Details
584-09-8 -
 Rubidium carbonate CAS 584-09-8View Details
Rubidium carbonate CAS 584-09-8View Details
584-09-8 -
 Rubidium carbonate CAS 584-09-8View Details
Rubidium carbonate CAS 584-09-8View Details
584-09-8 -
 Rubidium carbonate CAS 584-09-8View Details
Rubidium carbonate CAS 584-09-8View Details
584-09-8 -
 Rubidium CAS 584-09-8View Details
Rubidium CAS 584-09-8View Details
584-09-8 -
 Rubidium CAS 584-09-8View Details
Rubidium CAS 584-09-8View Details
584-09-8 -
 Rubidium carbonate 95% CAS 584-09-8View Details
Rubidium carbonate 95% CAS 584-09-8View Details
584-09-8