Rucaparib
- CAS NO.:283173-50-2
- Empirical Formula: C19H18FN3O
- Molecular Weight: 323.36
- MDL number: MFCD11977252
- EINECS: 814-445-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
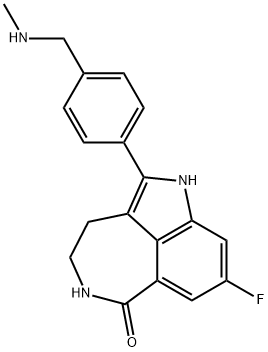
What is Rucaparib?
Absorption
Rucaparib exhibits a linear pharmacokinetic profile over the dose range from 240 mg to 840 mg twice daily. The mean (coefficient of variation [CV]) steady-state rucaparib Cmax is 1940 ng/mL (54%) and AUC0-12h is 16900 h x ng/mL (54%) at the approved recommended dosage. The mean AUC accumulation ratio is 3.5 to 6.2 fold. The median Tmax at the steady state is 1.9 hours, with a range of 0 to 5.98 hours at the approved recommended dosage. The mean absolute bioavailability is 36%, with a range of 30 to 45%.
A high-fat meal increased Cmax and AUC0-24h by 20% and 38%, respectively. The Tmax was delayed by 2.5 hours.
Toxicity
There is no information regarding the LD50 and overdose of rucaparib.
Description
Rucaparib was approved in the US as an oral treatment for advanced ovarian cancer. Development of rucaparib began with collaborations between Cancer Research UK and Agouron Pharmaceuticals (later acquired by Pfizer). Global development rights for rucaparib were ultimately granted to Clovis Oncology via a licensing agreement from Pfizer. To qualify for treatment with rucaparib monotherapy, patients must demonstrate deleterious breast cancer (BRCA) mutation (germline and/or somatic)- associated advanced ovarian cancer and also must have previously been treated with two or more chemotherapy regimens. Rucaparib functions as a small molecule poly(ADPribose) polymerase (PARP) inhibitor, which plays an important role in DNA repair. This newly approved drug displays nanomolar potency against PARP-1, -2, and -3 enzymes, which translates into improved efficacy over alternative therapies such as olaparib or niraparib. Furthermore, rucaparib is also known to cause vasodilation, which is thought to induce tumor perfusion and increased accumulation of the drug in cancer cells. Although rucaparib shows higher cytotoxicity in cancer cells with mutation of BRCA1/2 genes and other DNA repair genes, reduced tumor growth was observed in mouse xenograft models of human cancers with and without BRCA mutations.Rucaparib is also being pursued as a treatment for breast cancer and has displayed promising initial results in trials for pancreatic cancer.
Description
Poly(ADP-
The Uses of Rucaparib
Rucaparib is PARP1 inhibitor. It can be used in biological study of chemical screening to identify drugs that enhance or mitigate cellular responses to antibody-toxin fusion proteins using human B cell precursor leukemia cells and cervical adenocarcinoma cells.
Indications
Rucaparib is indicated for the maintenance treatment of adult patients with a deleterious BRCA mutation (germline and/or somatic)- associated recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in a complete or partial response to platinum-based chemotherapy.
Under accelerated approval by the FDA, rucaparib is also indicated for the treatment of adult patients with a deleterious BRCA mutation (germline and/or somatic)-associated metastatic castration-resistant prostate cancer (mCRPC) who have been treated with androgen receptor-directed therapy and a taxane-based chemotherapy.
Background
Rucaparib is an anticancer drug and poly (ADP-ribose) polymerase (PARP) inhibitor. PARP is an enzyme that plays an essential role in DNA repair. Rucaparib is proposed to work in several PARP-dependent and PARP-independent mechanisms of action; however, it causes a unique effect of synthetic lethality. By targeting the genetically-mutated cancer cells that lack a DNA repair mechanism, rucaparib causes cancer cell death and reduces tumour growth.
Rucaparib was granted FDA Breakthrough Therapy designation in April 2015 and accelerated approval in December 2016. The drug was later approved by the European Commission in May 2018. It is currently used to treat recurrent ovarian and prostate cancer in adults.
Definition
ChEBI: Rucaparib is a member of the class of azepinoindoles that is 1,3,4,5-tetrahydro-6H-azepino[5,4,3-cd]indol-6-one carrying additional 4-[(methylamino)methyl]phenyl and fluoro substituents at positions 2 and 8 respectively. It is an inhibitor of poly (ADP-ribose) polymerase and is used (as the camsylate salt) as monotherapy for advanced ovarian cancer and deleterious germline or somatic BRCA mutation. It has a role as an EC 2.4.2.30 (NAD(+) ADP-ribosyltransferase) inhibitor and an antineoplastic agent. It is an azepinoindole, a member of caprolactams, an organofluorine compound and a secondary amino compound. It is a conjugate base of a rucaparib(1+).
Pharmacokinetics
Rucaparib is an anticancer agent that exerts cytotoxic effects against cancer cells. It works by inhibiting poly (ADP-ribose) polymerase (PARP), an enzyme that plays a role in DNA repair. Rucaparib inhibits PARP-1, PARP-2, and PARP-3. It also interacts with PARP-4, PARP-10, PARP-12, PARP-15, and PARP-16, but to a lesser extent. In mice, rucaparib accumulated and was retained in tumours, inhibiting PARP enzymes for seven days.
Rucaparib decreases tumour growth in tumour cell lines with deficiencies in BRCA1/2 and other DNA repair genes. In addition to PARP inhibition, rucaparib demonstrated PARP-independent cytotoxic mechanisms in cancer cells. When co-administered with other chemotherapeutic agents, rucaparib contributed to synergistic or additive effects in vitro and in vivo. There is evidence that rucaparib can sensitize cancer cells to chemotherapy. Rucaparib can also cause vasodilation, which may increase tumour perfusion and enhance the accumulation of cytotoxic drugs in cancer cells.
Synthesis
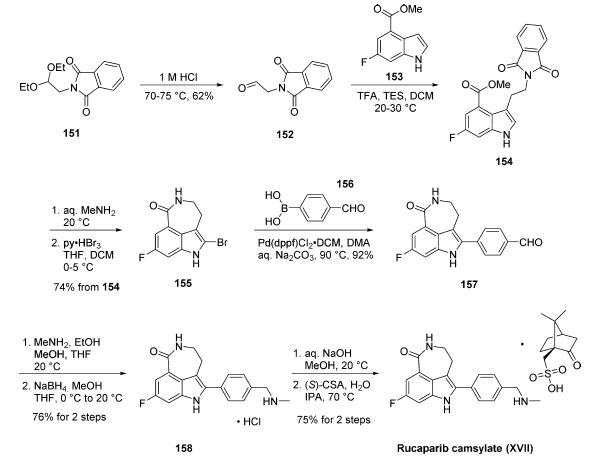
Synthesis of rucaparib camsylate begins from commercially available phthalimide acetal 151. Unveiling of the aldehyde via treatment with aqueous HCl and precipitation from toluene provided aldehyde 152 in 62% yield. To avoid polymerization, 152 was immediately subjected to 6-fluoro-1Hindole- 4-carboxylic acid methyl ester (153) under reductive conditions in the presence of acid to give rise to tryptamine derivative 154. After considerable research, optimal conditions for this transformation (triethylsilane in DCM/TFA) were found that were successful on up to 15.7 kg scale, enabling clean separation of the aldehyde reduction byproduct following crystallization. Conversion of the phthalimide within 154 to the corresponding amine using aqueous methylamine at room temperature was accompanied by an intramolecular cyclization reaction to secure the intermediate lactam as a solid in 89% isolated yield. This was followed by a high-yielding bromination reaction (83%) at the indole C-2 position employing pyridinium tribromide, providing access to indoloazepinone 155. After screening various catalysts for the coupling of bromide 155 and commercial boronic acid 156, Pd(dppf)Cl2?¤ DCM was found to reliably deliver the desired coupling product with reasonable rates of reaction. Thus, after development of an extensively optimized reaction protocol, Suzuki coupling of 155 and 4-formylphenylboronic acid (156) with Pd(dppf)Cl2?¤DCM and Na2CO3 in DMA at 90 ??C generated the desired 2-arylated indole 157 in high yield (92%) after trituration and reslurry with methanol. Conversion of aldehyde 157 to amine 158 necessitated a two-pot procedure designed to limit the formation of dimerization and aldehyde reduction products that generally arise under conventional onepot reductive amination conditions and have traditionally been problematic on scale. Toward this end, subjection of aldehyde 157 to a methylamine solution in EtOH/MeOH/THF and wash of the resulting reaction solids with methanol led to efficient isolation of pure imine intermediate, which could be immediately reduced with NaBH4 in THF/MeOH, providing hydrochloride salt 158 upon acidic workup in 76% over two steps. The two remaining steps for conversion to the drug involve a salt-swap, first breaking the HCl salt with NaOH in MeOH, then treatment with (S)-camphorsulfonic acid/IPA/ H2O at 70 ??C. Filtration and washing of the cake with water generated rucaparib camsylate (XVII) in 95% yield.
Metabolism
In vitro, rucaparib is primarily metabolized by CYP2D6 and, to a lesser extent, by CYP1A2 and CYP3A4. In addition to CYP-based oxidation, rucaparib also undergoes N-demethylation, N-methylation, and glucuronidation. In one study, seven metabolites of rucaparib were identified in plasma, urine, and feces.
Properties of Rucaparib
| Melting point: | 187 - 189°C |
| Boiling point: | 625.2±55.0 °C(Predicted) |
| Density | 1.281 |
| storage temp. | 2-8°C(protect from light) |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Yellow solid. |
| pka | 14.10±0.20(Predicted) |
| color | Pale Yellow to Yellow |
Safety information for Rucaparib
Computed Descriptors for Rucaparib
Rucaparib manufacturer
New Products
1-Amino-1-cyclohexanecarboxylic acid Cycloleucine 6-Bromo-3-iodo-1-methyl-1H-indazole 3-(2,4-Dimethoxybenzyl)dihydropyrimidine-2,4(1H,3H)-dione 7-Bromo-1H-indazole ELECTROLYTIC IRON POWDER 2-Methyl-2-phenylpropyl acetate 1-Aminocyclobutanecarboxylic acid 1-(2-Ethoxyethyl)-2-(piperidin-4-yl)-1H-benzo[d]imidazole hydrochloride Decanonitrile tert-butyl 4-(1H-benzo[d]iMidazol-2-yl)piperidine-1-carboxylate 4-Ethylbenzylamine Methyl 5-bromo-2-chloro-3-nitrobenzoate N-(5-Amino-2-methylphenyl)acetamide 2-Chloro-3-nitropyridine 5-Bromo-2,3-dimethoxypyridine methyl 6-chloro-2-(chloromethyl)nicotinate 2-methoxy-4-methyl-5-nitro pyridine 2-iodo-5-bromo pyridine 2-amino-4-methyl-5-nitro pyridine 5-Fluoro-2-Oxindole methyl L-alaninate hydrochloride diethyl L-glutamate hydrochloride Ethyl tosylcarbamateRelated products of tetrahydrofuran
You may like
-
 283173-50-2 Rucaparib 98%View Details
283173-50-2 Rucaparib 98%View Details
283173-50-2 -
 Rucaparib 99%View Details
Rucaparib 99%View Details -
 Ethoxymethylenemalononitrile 99% HPLCView Details
Ethoxymethylenemalononitrile 99% HPLCView Details
123-06-8 -
 Diethyl Disulfide 99% HPLCView Details
Diethyl Disulfide 99% HPLCView Details
110-81-6 -
 6285-05-8. 1-(4-chlorophenyl) propan-1-one 98%View Details
6285-05-8. 1-(4-chlorophenyl) propan-1-one 98%View Details
6285-05-8. -
 4-chloro-3,5-dinitropyridine 98%View Details
4-chloro-3,5-dinitropyridine 98%View Details -
 401-95-6 99% HPLCView Details
401-95-6 99% HPLCView Details
401-95-6 -
 171663-13-1 tert-Butyl 3-bromobenzylcarbamate 98%View Details
171663-13-1 tert-Butyl 3-bromobenzylcarbamate 98%View Details
171663-13-1