3-Aminobenzamide
Synonym(s):3-AB;3-ABA;3-Aminobenzamide;PARP Inhibitor I, 3-ABA - CAS 3544-24-9 - Calbiochem
- CAS NO.:3544-24-9
- Empirical Formula: C7H8N2O
- Molecular Weight: 136.15
- MDL number: MFCD00007989
- EINECS: 222-586-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-10 11:33:04
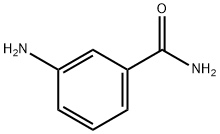
What is 3-Aminobenzamide?
Description
The poly(ADP-
Chemical properties
beige powder
The Uses of 3-Aminobenzamide
3-Aminobenzamide is used as an endogenous poly-ADP-ribosyltransferase inhibitor. Through its effects on PARPs, 3-amino benzamide causes telomere shortening and stimulates angiogenesis. It displays neuroprotective, anti-necrotic effects.
The Uses of 3-Aminobenzamide
The poly(ADP-ribose) polymerases (PARPs) comprise a family of enzymes which post-translationally modify proteins by poly(ADP-ribosyl)ation. Since some PARPs are activated by DNA strand breaks, PARP signaling has roles in DNA repair, apoptosis, inflammation, and other cellular responses. 3-amino Benzamide is an inhibitor of PARPs (Ki = 1.8 μM). Through its effects on PARPs, 3-amino benzamide causes telomere shortening and stimulates angiogenesis. It has PARP-mediated actions in such diverse diseases as atherosclerosis, neurogenesis, and cancer.
What are the applications of Application
3-Aminobenzamide is An inhibitor of PARP
Definition
ChEBI: A substituted aniline that is benzamide in which one of the meta- hydrogens is replaced by an amino group.
Biochem/physiol Actions
3-Aminobenzamide enhances cell death, unscheduled DNA synthesis and repair replication by interrupting the rejoining of DNA strand breaks induced by both ionizing radiations and several alkylating agents. It has an ability to inhibit the activity of nuclear enzyme poly (ADP-ribose) synthetase (PARS). 3-Aminobenzamide exhibits protective action against myocardial reperfusion injury and local inflammation.
References
1) Purnell and Whish (1980), Novel inhibitors of poly(ADP-ribose) synthetase; Biochem. J., 185 775 2) Kuo et al. (1996), Inhibitors of poly(ADP-ribose) polymerase block nitric oxide-induced apoptosis but not differentiation in human leukemia HL-60 cells; Biochem. Biophys. Res. Commun., 219 502 3) Malorni et al. (1995), 3-Aminobenzamide protects cells from UV-B-induced apoptosis by acting on cytoskeleton and substrate adhesion; Biochem. Biophys. Res. Commun., 207 715 4) Heller et al. (1995), Inactivation of the poly(ADP-ribose) polymerase gene affects oxygen radical and nitric oxide toxicity in islet cells; J. Biol. Chem., 270 11176
Properties of 3-Aminobenzamide
| Melting point: | 115-116 °C (lit.) |
| Boiling point: | 250.42°C (rough estimate) |
| Density | 1.1778 (rough estimate) |
| refractive index | 1.5769 (estimate) |
| storage temp. | room temp |
| solubility | ethanol: 50 mg/mL, clear, faintly yellow |
| form | powder |
| pka | 16.26±0.50(Predicted) |
| color | white to off-white |
| Water Solubility | Soluble in dimethylformamide (~30 mg/ml), dimethyl sulfoxide (~30 mg/ml), phosphate buffered saline (pH7.2) (~2 mg/ml), water (25 mg/ml), and ethanol (25 mg/ml). |
| Sensitive | Light Sensitive |
| BRN | 2802373 |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 1 month. |
| CAS DataBase Reference | 3544-24-9(CAS DataBase Reference) |
| NIST Chemistry Reference | 3-NH2-C6H4CONH2(3544-24-9) |
Safety information for 3-Aminobenzamide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for 3-Aminobenzamide
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 3544-24-9 3-Aminobenzamide 98%View Details
3544-24-9 3-Aminobenzamide 98%View Details
3544-24-9 -
 3-Aminobenzamide CAS 3544-24-9View Details
3-Aminobenzamide CAS 3544-24-9View Details
3544-24-9 -
 3-Aminobenzamide CAS 3544-24-9View Details
3-Aminobenzamide CAS 3544-24-9View Details
3544-24-9 -
 PARP Inhibitor I, 3-ABA CAS 3544-24-9View Details
PARP Inhibitor I, 3-ABA CAS 3544-24-9View Details
3544-24-9 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1