Rubidium chloride
Synonym(s):Rubidium chloride;Rubidium monochloride;Rubidium(I) chloride
- CAS NO.:7791-11-9
- Empirical Formula: ClRb
- Molecular Weight: 120.92
- MDL number: MFCD00011187
- EINECS: 232-240-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-18 19:10:02
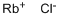
What is Rubidium chloride?
Chemical properties
Rubidium chloride is an alkali metal halide, chemical formula RbCl, white crystalline powder; lustrous. When heated it decrepitates, melts, and volatizes. soluble in water, slightly soluble in alcohol. In the gaseous state, RbCl is a diatomic molecule with a bond length of about 2.7868A. The bond length increases to 3.285?A in the cubic system, showing a high ionic coordination number in the solid state.
Rubidium chlorides is a source of rubidium metal. Rubidium is usually prepared by the thermal reduction of rubidium chloride.When rubidium chloride and calcium are heated together in stoichiometric proportions at 900-1000°C under a pressure of about 0.02 torr, rubidium distills over.
The Uses of Rubidium chloride
It is used in testing for perchloric acid in laboratories, and is a source of rubidium. Rubidium chloride is used as a catalyst and additive in petrol. It has been employed to prepare molecular nanowires for nanoscale devices. Thin layer of rubidium chloride enhance the attributes of organic light-emitting diodes. Rubidium chloride also plays an essential role for enhancement in the memory storage property. It is an excellent non-invasive biomarker, and used in biomedical research.
Definition
ChEBI: Rubidium chloride is an inorganic chloride composed of rubidium and chloride ions in a 1:1 ratio. It has a role as an antidepressant and a biomarker. It is a rubidium molecular entity and an inorganic chloride.
What are the applications of Application
Rubidium chloride (RbCl) , an alkali halide may be used to dope L-alanine hydrogen chloride monohydrate single crystals to alter its dielectric properties. Rubidium chloride has been used as a marker in studies on several herbivorous insects.
Rubidium chloride is used as a catalyst and additive in gasoline. In materials science, RbCl has been used to prepare molecular nanowires as potential precursors of nanoscale devices. In chromatography, RbCl and other univalent salts have been studied for their influence on the capillary electrophoretic separation of amino acids labeled with 3-(4-carboxybenzoyl)-quinoline-2-carboxaldehyde. RbCl is utilized in the transfection of bacteria. RbCl has been used to investigate the gating and permeability of ion channels produced by botulinum neurotoxin types A and E in membranes from cultured PC12 cells.
Toxicology
The chemical, physical and toxicological properties of rubidium chloride have not been thoroughly investigated and recorded. Acute Effects: Inhalation: May cause irritation to the respiratory system. Ingestion: May cause ataxia and hyperirritability.
Purification Methods
Crystallise it from water (0.7mL/g) by cooling to 0o from 100o. Its solubility in H2O is 77.3% at 0.6o, 90.3% at 70o and 147% at boiling point. [D.nges in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 951-955 1963.]
Properties of Rubidium chloride
| Melting point: | 715 °C (lit.) |
| Boiling point: | 1390 °C |
| Density | 2.8 g/mL at 25 °C (lit.) |
| refractive index | 1.493 |
| Flash point: | 1390°C |
| storage temp. | Store at +5°C to +30°C. |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | beads |
| color | White |
| Specific Gravity | 2.8 |
| PH | 5.0-8.0 (25℃, 1M in H2O) |
| Water Solubility | 910 g/L (20 ºC) |
| Sensitive | Hygroscopic |
| λmax | λ: 260 nm Amax: 0.03 λ: 280 nm Amax: 0.03 |
| Merck | 14,8284 |
| Dielectric constant | 4.9400000000000004 |
| Stability: | Stable. Incompatible with bromine trifluoride, strong acids. Hygroscopic - protect from moisture. |
| CAS DataBase Reference | 7791-11-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Rubidium chloride(7791-11-9) |
| EPA Substance Registry System | Rubidium chloride (RbCl) (7791-11-9) |
Safety information for Rubidium chloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H303:Acute toxicity,oral |
| Precautionary Statement Codes |
P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P403:Store in a well-ventilated place. |
Computed Descriptors for Rubidium chloride
| InChIKey | FGDZQCVHDSGLHJ-UHFFFAOYSA-M |
Rubidium chloride manufacturer
JSK Chemicals
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran



You may like
-
 Rubidium chloride CAS 7791-11-9View Details
Rubidium chloride CAS 7791-11-9View Details
7791-11-9 -
 Rubidium chloride CAS 7791-11-9View Details
Rubidium chloride CAS 7791-11-9View Details
7791-11-9 -
 Rubidium chloride CAS 7791-11-9View Details
Rubidium chloride CAS 7791-11-9View Details
7791-11-9 -
 Rubidium chloride CAS 7791-11-9View Details
Rubidium chloride CAS 7791-11-9View Details
7791-11-9 -
 7791-11-9 Rubidium chloride, 99% 99%View Details
7791-11-9 Rubidium chloride, 99% 99%View Details
7791-11-9 -
 Rubidium chloride, GR 99.8% CAS 7791-11-9View Details
Rubidium chloride, GR 99.8% CAS 7791-11-9View Details
7791-11-9 -
 Rubidium chloride CAS 7791-11-9View Details
Rubidium chloride CAS 7791-11-9View Details
7791-11-9 -
 Rubidium chloride CAS 7791-11-9View Details
Rubidium chloride CAS 7791-11-9View Details
7791-11-9
