Nickel(II) chloride hexahydrate
Synonym(s):Nickel chloride hexahydrate 2;Nickel dichloride;Nickel dichloride hexahydrate;Nickel(II) chloride hexahydrate
- CAS NO.:7791-20-0
- Empirical Formula: Cl2Ni.6H2O
- Molecular Weight: 237.69
- MDL number: MFCD00149809
- EINECS: 616-576-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:17
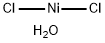
What is Nickel(II) chloride hexahydrate?
Chemical properties
Green solid
The Uses of Nickel(II) chloride hexahydrate
Nickel(II) chloride hexahydrate has been used:
- in the preparation of nickel platting solution for galvanostatically coating nickel inside the reduced carbon layer during the synthesis of multiwall carbon nanotube micromotors
- in screening of metals for inflammatory effect on vascular endothelial cells
- as an additive in artificial cerebrospinal fluid (ACSF) to analyse excitatory post synaptic currents (EPSPs) in the hippocampus slices
The Uses of Nickel(II) chloride hexahydrate
Anhydr salt as absorbent for NH3 in gas masks. Hexahydrate for nickel electroplating; manufacture of nickel catalysts.
The Uses of Nickel(II) chloride hexahydrate
Nickel(II) chloride hexahydrate may be employed as a catalyst in the high yield synthesis of tetra-substituted pyrrole derivatives. It may be used for the conversion of tetrahydropyranyl (THP) and tert-butyldimethylsilyl (TBS) ethers to the corresponding hydroxyl compounds.
What are the applications of Application
Nickel(II) chloride hexahydrate is a reagent used in electroplating and in gas masks to absorb NH3
Definition
ChEBI: A hydrate of nickel chloride containing nickel (in the +2 oxidation state), chloride and water moeities in the ratio 1:2:6.
General Description
Nickel(II) chloride hexahydrate is a nickel salt that can be used as a catalyst. It is cost effective and can be used in a variety of industrial processes. It can also be used in cross-coupling reactions.
Hazard
Confirmed carcinogen.
Biochem/physiol Actions
Nickel (Ni) is an essential trace element that plays an important role in the structure and function of DNA, RNA and protein. It has toxic effects on renal, pulmonary and cardiovascular system at higher concentrations. Nickel is implicated in carcinogenesis. Ni2+ ions is a clastogenic agent. Nickel ions (Ni2+) is a non-specific voltage-gated calcium channel blocker.
Safety Profile
Confirmed human carcinogen. Poison by intraperitoneal and intravenous routes. Experimental reproductive effects. Mutation data reported. Violent reaction with potassium. When heated to decomposition it emits very toxic fumes of Cl-. See also NICKEL COMPOUNDS and CHLORIDES.
Purification Methods
It crystallises from dilute HCl to form the green hexahydrate. At 70o this dehydrates to the tetrahydrate, and at higher temperatures it forms the anhydrous salt. It sublimes in yellow hexagonal scales in a stream of HCl. Store it in a desiccator as it is deliquescent. [Hart & Partington J Chem Soc 104 1943.]
Properties of Nickel(II) chloride hexahydrate
| Melting point: | 140℃ -H{2}O |
| Density | 1,92 g/cm3 |
| storage temp. | Store below +30°C. |
| solubility | 2540g/l |
| form | Solution |
| color | Green |
| PH | 4.9 (100g/l, H2O, 20℃) |
| Water Solubility | 2540 G/L (20 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,6505 |
| Exposure limits | ACGIH: TWA 0.1 mg/m3 NIOSH: IDLH 10 mg/m3; TWA 0.015 mg/m3 |
| Stability: | Stable. Hygroscopic. Incompatible with peroxides. |
| CAS DataBase Reference | 7791-20-0(CAS DataBase Reference) |
| EPA Substance Registry System | Nickel chloride (NiCl2), hexahydrate (7791-20-0) |
Safety information for Nickel(II) chloride hexahydrate
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H317:Sensitisation, Skin H334:Sensitisation, respiratory H341:Germ cell mutagenicity H372:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for Nickel(II) chloride hexahydrate
| InChIKey | LAIZPRYFQUWUBN-UHFFFAOYSA-L |
Nickel(II) chloride hexahydrate manufacturer
JSK Chemicals
Techno Pharmchem
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 Nickel chloride hexahydrate 98%View Details
Nickel chloride hexahydrate 98%View Details -
 Nickel(II) chloride hexahydrate CAS 7791-20-0View Details
Nickel(II) chloride hexahydrate CAS 7791-20-0View Details
7791-20-0 -
 Nickel(II) chloride hexahydrate CAS 7791-20-0View Details
Nickel(II) chloride hexahydrate CAS 7791-20-0View Details
7791-20-0 -
 Nickel(II) chloride hexahydrate CAS 7791-20-0View Details
Nickel(II) chloride hexahydrate CAS 7791-20-0View Details
7791-20-0 -
 Nickel(II) chloride hexahydrate CAS 7791-20-0View Details
Nickel(II) chloride hexahydrate CAS 7791-20-0View Details
7791-20-0 -
 Nickel (II) Chloride Hexahydrate pure CAS 7791-20-0View Details
Nickel (II) Chloride Hexahydrate pure CAS 7791-20-0View Details
7791-20-0 -
 Nickel chloride hexahydrate 98% AR CAS 7791-20-0View Details
Nickel chloride hexahydrate 98% AR CAS 7791-20-0View Details
7791-20-0 -
 Nickel(II) Chloride hexahydrate 98% CAS 7791-20-0View Details
Nickel(II) Chloride hexahydrate 98% CAS 7791-20-0View Details
7791-20-0
