Lanthanum(III) chloride
Synonym(s):Lanthanum trichloride
- CAS NO.:10099-58-8
- Empirical Formula: Cl3La
- Molecular Weight: 245.26
- MDL number: MFCD00011068
- EINECS: 233-237-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-18 22:22:14
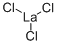
What is Lanthanum(III) chloride?
Chemical properties
Lanthanum(III) chloride is white powder or colourless crystals
The Uses of Lanthanum(III) chloride
Lanthanum chloride is used to prepare other lanthanum salts. The anhydrous chloride is employed to produce lanthanum metal.
The Uses of Lanthanum(III) chloride
Lanthanum chloride is a precursor for synthesis of lanthanum phosphate nano rods and used in gamma detectors. It is also used as a catalyst for high pressure oxidative chlorination of methane to chloromethane with hydrochloric acid and oxygen. In organic synthesis, lanthanum trichloride acts as lewis acid for the conversion of aldehydes to acetals.
Preparation
The heptahydrate is formed by dissolving the oxide, hydroxide or carbonate in hydrochloric acid, followed by crystallization. The anhydrous chloride is obtained by heating oxide, hydroxide, or carbonate in an atmosphere of dry hydrogen chloride.
La2(CO3)3 + 6HCl → 2LnCl3 + 3CO2 + 3H2O
Another method involves heating lanthanum oxide with excess ammonium chloride at 300°C.
General Description
Lanthanum(III) chloride is white crystalline solid. Gives an amber aqueous solution that can cause destruction or irreversible alterations in human skin tissue at the site of contact. Has a severe corrosion rate on steel.
Air & Water Reactions
Deliquescent. Water soluble.
Reactivity Profile
Aqueous solutions of LANTHANUM CHLORIDE contain moderate concentrations of hydrogen ions and react as acids to neutralize bases. Does not usually react as either oxidizing agents or reducing agents but such behavior is not impossible. May catalyze organic reactions.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Properties of Lanthanum(III) chloride
| Melting point: | 860 °C(lit.) |
| Boiling point: | 1812 °C(lit.) |
| Density | 3.84 g/mL at 25 °C(lit.) |
| Flash point: | 1000°C |
| storage temp. | Inert atmosphere,Room Temperature |
| form | beads |
| color | White to almost white |
| Specific Gravity | 3.842 |
| Water Solubility | Soluble in water. |
| Sensitive | Hygroscopic |
| Merck | 14,5363 |
| Stability: | Stability |
| CAS DataBase Reference | 10099-58-8(CAS DataBase Reference) |
| EPA Substance Registry System | Lanthanum chloride (LaCl3) (10099-58-8) |
Safety information for Lanthanum(III) chloride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H290:Corrosive to Metals H317:Sensitisation, Skin H318:Serious eye damage/eye irritation H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P234:Keep only in original container. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Lanthanum(III) chloride
Lanthanum(III) chloride manufacturer
JSK Chemicals
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
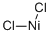


You may like
-
 Lanthanum (III) chloride, anhydrous 99%View Details
Lanthanum (III) chloride, anhydrous 99%View Details -
 Lanthanum(III) chloride, Ultra dry CAS 10099-58-8View Details
Lanthanum(III) chloride, Ultra dry CAS 10099-58-8View Details
10099-58-8 -
 Lanthanum(III) chloride, Ultra dry CAS 10099-58-8View Details
Lanthanum(III) chloride, Ultra dry CAS 10099-58-8View Details
10099-58-8 -
 Lanthanum(III) chloride, Ultra dry CAS 10099-58-8View Details
Lanthanum(III) chloride, Ultra dry CAS 10099-58-8View Details
10099-58-8 -
 Lanthanum(III) chloride, Ultra dry CAS 10099-58-8View Details
Lanthanum(III) chloride, Ultra dry CAS 10099-58-8View Details
10099-58-8 -
 Lanthanum(III) chloride, Ultra dry CAS 10099-58-8View Details
Lanthanum(III) chloride, Ultra dry CAS 10099-58-8View Details
10099-58-8 -
 Lanthanum(III) chloride, Ultra dry CAS 10099-58-8View Details
Lanthanum(III) chloride, Ultra dry CAS 10099-58-8View Details
10099-58-8 -
 Lanthanum chloride CAS 10099-58-8View Details
Lanthanum chloride CAS 10099-58-8View Details
10099-58-8
