Ivosidenib
- CAS NO.:1448347-49-6
- Empirical Formula: C28H22ClF3N6O3
- Molecular Weight: 582.96
- MDL number: MFCD29036964
- Update Date: 2024-11-19 15:53:33
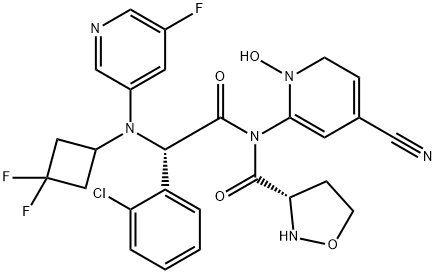
What is Ivosidenib?
Absorption
Following oral administration, ivosidenib is rapidly absorbed. The Cmax following a single oral dose is 4503 ng/mL in patients with relapsed or refractory AML, 4820 ng/mL in patients with newly diagnosed AML who were also treated with azacitidine, and 4060 ng/mL in patients with cholangiocarcinoma. The steady-state was reached within 14 days. The steady-state Cmax is 6551 ng/mL in patients with relapsed or refractory AML, 6145 ng/mL in patients with newly diagnosed AML who were also treated with azacitidine, and 4799 ng/mL in patients with cholangiocarcinoma. The Tmax ranges from two to three hours.
A high-fat meal increases ivosidenib exposure.
Toxicity
There is limited information regarding the LD50 or overdose of ivosidenib.
Ivosidenib is associated with a risk of differentiation syndrome, Guillain-Barre syndrome, and embryo-fetal toxicity.
Description
AG-120 is an orally available inhibitor of isocitrate dehydrogenase type 1 (IDH1) that specifically inhibits a mutated form of IDH1 in the cytoplasm, preventing the formation of 2-hydroxyglutarate. AG-120 may induce cellular differentiation as well as inhibit proliferation in IDH1-expressing tumor cells.
The Uses of Ivosidenib
Ivosidenib is a first-in-class mutant IDH1 inhibitor for the treatment of IDH1 mutant cancers.
Indications
Ivosidenib is an isocitrate dehydrogenase-1 (IDH1) inhibitor approved for use in the US and Europe. It is indicated for the treatment of patients with a susceptible IDH1 mutation with:
Background
Ivosidenib is a first-in-class isocitrate dehydrogenase-1 (IDH1) inhibitor. IDH1 is an enzyme that is often mutated and overexpressed in some cancers, leading to aberrant cell growth and proliferation. Ivosidenib inhibits mutated IDH1, blocking the enzymatic activity and further differentiation of cancer cells.
Ivosidenib was granted accelerated approval by the FDA in July 2018 for the treatment of relapsed of refractory acute myeloid leukemia in adults. It is currently approved to also treat newly diagnosed acute myeloid leukemia in older adults in combination azacitidine or as monotherapy, as well as locally advanced or metastatic cholangiocarcinoma in adults. The drug is only effective in patients with a susceptible IDH1 mutation.
In February 2023, the EMA's Committee for Medicinal Products for Human Use (CHMP) adopted a positive opinion of ivosidenib and recommended it be granted marketing authorization for the treatment of acute myeloid leukemia and cholangiocarcinoma. It was fully approved by the EMA in May 2023.
Definition
ChEBI: Ivosidenib is a tertiary carboxamide resulting from the formal condensation of the carboxy group of (2S)-1-(4-cyanopyridin-2-yl)-5-oxopyrrolidine-2-carboxylic acid with the secondary amino group of (2S)-2-(2-chlorophenyl)-N-(3,3-difluorocyclobutyl)-2-[(5-fluoropyridin-3-yl)amino]acetamide. It is approved by the FDA for the treatment of acute myeloid leukemia (AML) in patients with an isocitrate dehydrogenase-1 (IDH1) mutation. It has a role as an antineoplastic agent and an EC 1.1.1.42 (isocitrate dehydrogenase) inhibitor. It is a member of monochlorobenzenes, a cyanopyridine, a member of pyrrolidin-2-ones, an organofluorine compound, a tertiary carboxamide and a secondary carboxamide.
Pharmacokinetics
Ivosidenib is an antineoplastic agent that is effective in cancers with a susceptible IDH1 mutation, which indicates increased levels of oncometabolite D-2-hydroxyglutarate (D-2HG) in cancer cells. Ivosidenib decreases D-2HG levels in a dose-dependent manner by inhibiting the IDH1 enzyme. Ivosidenib inhibits both the mutant and wild-type IDH1 but does not inhibit IDH2.
in vitro
tf-1 cells or primary human aml patient samples expressing mutant idh1 were treated with ag-120, and the results showed that in tf-1 idh1-r132h cells, ag-120 was able to decrease the intracellular 2-hg levels, inhibit growth factor independent proliferation and restore erythropoietin-induced differentiation [1].
Metabolism
Ivosidenib is predominantly metabolized by CYP3A4 via oxidation. The exact chemical structures of the metabolites formed from CYP3A4-mediated oxidation have not been fully characterized. Ivosidenib can also undergo N-dealkylation and hydrolysis as minor metabolic pathways.
storage
Store at -20°C
References
[1] erica hansen et al. ag-120, an oral, selective, first-in-class, potent inhibitor of mutant idh1, reduces intracellular 2hg and induces cellular differentiation in tf-1 r132h cells and primary human idh1 mutant aml patient samples treated ex vivo. blood 2014 124:3734.
[2] http://www. aacr.org/newsroom/pages/news-release-detail.aspx itemid=789#.wlauom997iu
Properties of Ivosidenib
| Melting point: | 151-173°C |
| Boiling point: | 854.3±65.0 °C(Predicted) |
| Density | 1.51±0.1 g/cm3(Predicted) |
| storage temp. | -20°C Freezer, Under inert atmosphere |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 11.58±0.40(Predicted) |
| color | White to Off-White |
Safety information for Ivosidenib
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Ivosidenib
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
![(S)-tert-butyl 2-(4-(4-chlorophenyl)-2,3,9-triMethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)acetate](https://img.chemicalbook.in/CAS/GIF/1268524-70-4.gif)
You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4